Phase Separation Phenomena in Solutions of Polysulfone in Mixtures of a Solvent and a Nonsolvent: Relationship with Membrane Formation*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modelling and Numerical Simulation of Phase Separation in Polymer Modified Bitumen by Phase- Field Method
http://www.diva-portal.org Postprint This is the accepted version of a paper published in Materials & design. This paper has been peer- reviewed but does not include the final publisher proof-corrections or journal pagination. Citation for the original published paper (version of record): Zhu, J., Lu, X., Balieu, R., Kringos, N. (2016) Modelling and numerical simulation of phase separation in polymer modified bitumen by phase- field method. Materials & design, 107: 322-332 http://dx.doi.org/10.1016/j.matdes.2016.06.041 Access to the published version may require subscription. N.B. When citing this work, cite the original published paper. Permanent link to this version: http://urn.kb.se/resolve?urn=urn:nbn:se:kth:diva-188830 ACCEPTED MANUSCRIPT Modelling and numerical simulation of phase separation in polymer modified bitumen by phase-field method Jiqing Zhu a,*, Xiaohu Lu b, Romain Balieu a, Niki Kringos a a Department of Civil and Architectural Engineering, KTH Royal Institute of Technology, Brinellvägen 23, SE-100 44 Stockholm, Sweden b Nynas AB, SE-149 82 Nynäshamn, Sweden * Corresponding author. Email: [email protected] (J. Zhu) Abstract In this paper, a phase-field model with viscoelastic effects is developed for polymer modified bitumen (PMB) with the aim to describe and predict the PMB storage stability and phase separation behaviour. The viscoelastic effects due to dynamic asymmetry between bitumen and polymer are represented in the model by introducing a composition-dependent mobility coefficient. A double-well potential for PMB system is proposed on the basis of the Flory-Huggins free energy of mixing, with some simplifying assumptions made to take into account the complex chemical composition of bitumen. -

Phase Diagrams and Phase Separation
Phase Diagrams and Phase Separation Books MF Ashby and DA Jones, Engineering Materials Vol 2, Pergamon P Haasen, Physical Metallurgy, G Strobl, The Physics of Polymers, Springer Introduction Mixing two (or more) components together can lead to new properties: Metal alloys e.g. steel, bronze, brass…. Polymers e.g. rubber toughened systems. Can either get complete mixing on the atomic/molecular level, or phase separation. Phase Diagrams allow us to map out what happens under different conditions (specifically of concentration and temperature). Free Energy of Mixing Entropy of Mixing nA atoms of A nB atoms of B AM Donald 1 Phase Diagrams Total atoms N = nA + nB Then Smix = k ln W N! = k ln nA!nb! This can be rewritten in terms of concentrations of the two types of atoms: nA/N = cA nB/N = cB and using Stirling's approximation Smix = -Nk (cAln cA + cBln cB) / kN mix S AB0.5 This is a parabolic curve. There is always a positive entropy gain on mixing (note the logarithms are negative) – so that entropic considerations alone will lead to a homogeneous mixture. The infinite slope at cA=0 and 1 means that it is very hard to remove final few impurities from a mixture. AM Donald 2 Phase Diagrams This is the situation if no molecular interactions to lead to enthalpic contribution to the free energy (this corresponds to the athermal or ideal mixing case). Enthalpic Contribution Assume a coordination number Z. Within a mean field approximation there are 2 nAA bonds of A-A type = 1/2 NcAZcA = 1/2 NZcA nBB bonds of B-B type = 1/2 NcBZcB = 1/2 NZ(1- 2 cA) and nAB bonds of A-B type = NZcA(1-cA) where the factor 1/2 comes in to avoid double counting and cB = (1-cA). -

Partition Coefficients in Mixed Surfactant Systems
Partition coefficients in mixed surfactant systems Application of multicomponent surfactant solutions in separation processes Vom Promotionsausschuss der Technischen Universität Hamburg-Harburg zur Erlangung des akademischen Grades Doktor-Ingenieur genehmigte Dissertation von Tanja Mehling aus Lohr am Main 2013 Gutachter 1. Gutachterin: Prof. Dr.-Ing. Irina Smirnova 2. Gutachterin: Prof. Dr. Gabriele Sadowski Prüfungsausschussvorsitzender Prof. Dr. Raimund Horn Tag der mündlichen Prüfung 20. Dezember 2013 ISBN 978-3-86247-433-2 URN urn:nbn:de:gbv:830-tubdok-12592 Danksagung Diese Arbeit entstand im Rahmen meiner Tätigkeit als wissenschaftliche Mitarbeiterin am Institut für Thermische Verfahrenstechnik an der TU Hamburg-Harburg. Diese Zeit wird mir immer in guter Erinnerung bleiben. Deshalb möchte ich ganz besonders Frau Professor Dr. Irina Smirnova für die unermüdliche Unterstützung danken. Vielen Dank für das entgegengebrachte Vertrauen, die stets offene Tür, die gute Atmosphäre und die angenehme Zusammenarbeit in Erlangen und in Hamburg. Frau Professor Dr. Gabriele Sadowski danke ich für das Interesse an der Arbeit und die Begutachtung der Dissertation, Herrn Professor Horn für die freundliche Übernahme des Prüfungsvorsitzes. Weiterhin geht mein Dank an das Nestlé Research Center, Lausanne, im Besonderen an Herrn Dr. Ulrich Bobe für die ausgezeichnete Zusammenarbeit und der Bereitstellung von LPC. Den Studenten, die im Rahmen ihrer Abschlussarbeit einen wertvollen Beitrag zu dieser Arbeit geleistet haben, möchte ich herzlichst danken. Für den außergewöhnlichen Einsatz und die angenehme Zusammenarbeit bedanke ich mich besonders bei Linda Kloß, Annette Zewuhn, Dierk Claus, Pierre Bräuer, Heike Mushardt, Zaineb Doggaz und Vanya Omaynikova. Für die freundliche Arbeitsatmosphäre, erfrischenden Kaffeepausen und hilfreichen Gespräche am Institut danke ich meinen Kollegen Carlos, Carsten, Christian, Mohammad, Krishan, Pavel, Raman, René und Sucre. -

Chapter 9: Other Topics in Phase Equilibria
Chapter 9: Other Topics in Phase Equilibria This chapter deals with relations that derive in cases of equilibrium between combinations of two co-existing phases other than vapour and liquid, i.e., liquid-liquid, solid-liquid, and solid- vapour. Each of these phase equilibria may be employed to overcome difficulties encountered in purification processes that exploit the difference in the volatilities of the components of a mixture, i.e., by vapour-liquid equilibria. As with the case of vapour-liquid equilibria, the objective is to derive relations that connect the compositions of the two co-existing phases as functions of temperature and pressure. 9.1 Liquid-liquid Equilibria (LLE) 9.1.1 LLE Phase Diagrams Unlike gases which are miscible in all proportions at low pressures, liquid solutions (binary or higher order) often display partial immiscibility at least over certain range of temperature, and composition. If one attempts to form a solution within that certain composition range the system splits spontaneously into two liquid phases each comprising a solution of different composition. Thus, in such situations the equilibrium state of the system is two phases of a fixed composition corresponding to a temperature. The compositions of two such phases, however, change with temperature. This typical phase behavior of such binary liquid-liquid systems is depicted in fig. 9.1a. The closed curve represents the region where the system exists Fig. 9.1 Phase diagrams for a binary liquid system showing partial immiscibility in two phases, while outside it the state is a homogenous single liquid phase. Take for example, the point P (or Q). -

Vapor Condensation Under Nonequilibrium Conditions Victor Henry Heiskala Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1963 Vapor condensation under nonequilibrium conditions Victor Henry Heiskala Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Chemical Engineering Commons, and the Nuclear Engineering Commons Recommended Citation Heiskala, Victor Henry, "Vapor condensation under nonequilibrium conditions " (1963). Retrospective Theses and Dissertations. 2935. https://lib.dr.iastate.edu/rtd/2935 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been 64—3956 microfilmed exactly as received HEISKALA, Victor Henry, 1938- VAPOR CONDENSATION UNDER NONEQUILIBRIUM CONDITIONS. Iowa State University of Science and Technology Ph.D., 1963 Engineering, chemical University Microfilms, Inc., Ann Arbor, Michigan VAPOR CONDENSATION UNDER NONEQUI LIBRIUM CONDITIONS ty Victor Eenry Heiskala A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Nuclear Engineering Approved: Signature was redacted for privacy. In Charge of Ms^ Work Signature was redacted for privacy. Signature was redacted for privacy. Iowa State University Of Science and Technology Ames, Iowa 1963 ii TABLE OP CONTENTS Page I. INTRODUCTION 1 II. REVIEW OF LITERATURE "3 A. Liquid Drop Theory 3 B. Constant Number Theory 7 C. Statistical Mechanical Theory 8 D. Other Theories 9 III., THE THEORY 11 A. -

Facile Fabrication, Properties and Application of Novel Thermo-Responsive Hydrogel
Home Search Collections Journals About Contact us My IOPscience Facile fabrication, properties and application of novel thermo-responsive hydrogel This article has been downloaded from IOPscience. Please scroll down to see the full text article. 2011 Smart Mater. Struct. 20 075005 (http://iopscience.iop.org/0964-1726/20/7/075005) View the table of contents for this issue, or go to the journal homepage for more Download details: IP Address: 143.89.188.2 The article was downloaded on 01/06/2011 at 02:21 Please note that terms and conditions apply. IOP PUBLISHING SMART MATERIALS AND STRUCTURES Smart Mater. Struct. 20 (2011) 075005 (7pp) doi:10.1088/0964-1726/20/7/075005 Facile fabrication, properties and application of novel thermo-responsive hydrogel Jiaxing Li1,2, Xiuqing Gong1,XinYi1, Ping Sheng1 and Weijia Wen1 1 Department of Physics and Institute of Nano Science and Technology, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong 2 Key Laboratory of Novel Thin Film Solar Cells, Institute of Plasma Physics, Chinese Academy of Sciences, PO Box 1126, 230031, Hefei, People’s Republic of China E-mail: [email protected] Received 21 February 2011, in final form 5 May 2011 Published 31 May 2011 Online at stacks.iop.org/SMS/20/075005 Abstract The authors report a novel thermo-responsive hydrogel design based on the thermally induced aggregation of two kinds of nonionic surfactants: triblock copolymer poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (EPE) and 4-octylphenol polyethoxylate (TX-100). In the preparation of the hydrogel, agarose is used to host the aqueous surfactant molecules, allowing them to move freely through its intrinsic network structure. -
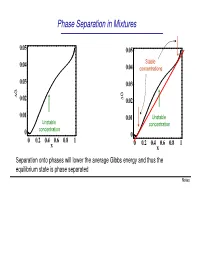
Phase Separation in Mixtures
Phase Separation in Mixtures 0.05 0.05 0.04 Stable 0.04 concentrations 0.03 0.03 G G ∆ 0.02 ∆ 0.02 0.01 0.01 Unstable Unstable concentration concentration 0 0 0 0.2 0.4 0.6 0.8 1 0 0.2 0.4 0.6 0.8 1 x x Separation onto phases will lower the average Gibbs energy and thus the equilibrium state is phase separated Notes Phase Separation versus Temperature Note that at higher temperatures the region of concentrations where phase separation takes place shrinks and G eventually disappears T increases ∆G = ∆U −T∆S This is because the term -T∆S becomes large at high temperatures. You can say that entropy “wins” over the potential energy cost at high temperatures Microscopically, the kinetic energy becomes x much larger than potential at high T, and the 1 x2 molecules randomly “run around” without noticing potential energy and thus intermix Oil and water mix at high temperature Notes Liquid-Gas Phase Separation in a Mixture A binary mixture can exist in liquid or gas phases. The liquid and gas phases have different Gibbs potentials as a function of mole fraction of one of the components of the mixture At high temperatures the Ggas <Gliquid because the entropy of gas is greater than that of liquid Tb2 T At lower temperatures, the two Gibbs b1 potentials intersect and separation onto gas and liquid takes place. Notes Boiling of a binary mixture Bubble point curve Dew point If we have a liquid at pint A that contains F A gas curve a molar fraction x1 of component 1 and T start heating it: E D - First the liquid heats to a temperature T’ C at constant x A and arrives to point B. -
Liquid-Liquid Phase Separation in Concentrated Solutions of Non-Crystallizable Polymers by Spinodal Decomposition by C
Kolloid-Z. u. Z. Polymere 243, 14-20 (1971 ) From the AKZO Research Laboratories Arnhem (The ~Vetherlands) Liquid-liquid phase separation in concentrated solutions of non-crystallizable polymers by spinodal decomposition By C. A. Smolders, J. J. van Aartsen, and A. Steenbergen With 11 figures (Received June 15, 1970) Introduction We will describe here under what condi- In the past, investigations of phase separa- tions spinodal decomposition can be expect- tion in concentrated polymer solutions cen- ed for polymer solutions. A number of ob- tered first of all on the crystallization kinet- servations on phase separation in concen- ics (1) and morphology (2) of polymer crys- trated solutions of poly(2,6-dimethyl-l,4- tals growing from solutions and, secondly, phenylene ether), (PPO| Tg = 210 ~ and on the equilibrium thermodynamic proper- of an ethylene-vinyl acetate copolymer ties of liquid-liquid phase separation phe- (Elvax-40), Tg ~ 0 ~ will be reported, which nomena (3). will make it clear that spinodal decompo- Papkov and Yefimova (4) have recently sition can be realized in polymer solutions. given a classification of different types of phase separation for polymer solutions, essen- Thermodynamic considerations tially the two types mentioned above, in which they also pay some attention to the Fig. 1 a and b, gives a schematic represen- difference in final state of separation (i. e. tation of the thermodynamics of liquid- "gel" or two liquid layers) and to the mechanism of separation itself. For liquid- A Fm [~_ liquid phase separation of solutions of non- crystallizable polymers the difference in I LE -r final state is ascribed to a combined effect of the thermodynamics of the system (the width of the miscibility gap in the temperature-com- position diagram) and the flow properties of the concentrated polymer phase. -
Pak 2016, Sequence Determinants of Intracellular Phase Separation By
Article Sequence Determinants of Intracellular Phase Separation by Complex Coacervation of a Disordered Protein Graphical Abstract Authors Chi W. Pak, Martyna Kosno, Alex S. Holehouse, ..., David R. Liu, Rohit V. Pappu, Michael K. Rosen Correspondence [email protected] (R.V.P.), [email protected] (M.K.R.) In Brief Pak et al. describe cellular liquid-liquid phase separation of a negatively charged intrinsically disordered protein, the Nephrin intracellular domain. Phase separation is driven by co-assembly with positively charged partners, a process termed complex coacervation. Disordered regions with NICD-like sequence features are common in the human proteome, suggesting complex coacervation may be widespread. Highlights d Disordered Nephrin intracellular domain (NICD) forms phase- separated nuclear bodies d NICD phase separates via complex coacervation d Aromatic/hydrophobic residues and high (À) charge density promote phase separation d Disordered regions with NICD-like sequence features are common in human proteome Pak et al., 2016, Molecular Cell 63, 72–85 July 7, 2016 ª 2016 Elsevier Inc. http://dx.doi.org/10.1016/j.molcel.2016.05.042 Molecular Cell Article Sequence Determinants of Intracellular Phase Separation by Complex Coacervation of a Disordered Protein Chi W. Pak,1 Martyna Kosno,1 Alex S. Holehouse,2,3 Shae B. Padrick,1 Anuradha Mittal,3 Rustam Ali,1 Ali A. Yunus,1 David R. Liu,4 Rohit V. Pappu,3,* and Michael K. Rosen1,* 1Department of Biophysics and Howard Hughes Medical Institute, UT Southwestern Medical Center, Dallas, TX 75390, USA 2Computational and Molecular Biophysics Graduate Program, Washington University in St. Louis, St. Louis, MO 63130, USA 3Department of Biomedical Engineering and Center for Biological Systems Engineering, Washington University in St. -

Triton X-114 and SRP
FUNDAMENTAL STUDIES AND POTENTIAL APPLICATIONS OF CLOUD POINT EXTRACTION By MELISSA ENSOR FREIDERICH A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY WASHINGTON STATE UNIVERSITY Department of Chemistry DECEMBER 2011 To the Faculty of Washington State University: The members of the Committee appointed to examine the dissertation of MELISSA ENSOR FREIDERICH find it satisfactory and recommend that it be accepted. ___________________________________ Kenneth L. Nash, Ph.D., Chair ___________________________________ Sue B. Clark, Ph.D. ___________________________________ James O. Schenk, Ph.D. ___________________________________ Glenn Fugate, Ph.D. ii ACKNOWLEDGMENT “There are two possible outcomes: if the result confirms the hypothesis, then you've made a measurement. If the result is contrary to the hypothesis, then you've made a discovery.” Enrico Fermi “Failure is simply the opportunity to begin again, this time more intelligently.” Henry Ford “A man who carries a cat by the tail learns something he can learn in no other way.” Mark Twain The preceding quotes, to me, sum up my graduate school experience. The past five years have not been easy and often times seemed impossible. I need to acknowledge and thank the people who helped me get to where I am today because without them I would not have completed this journey. First of all, I want to thank my husband, John. He has been my rock and sometimes only friend and source of comfort during my time in Pullman. John never wavered in his support of me and usually believed in me more than I believed in myself. I would not have completed my Ph.D. -

Low Cloud Point Biodiesel 9/4/2014
Shanti Johnson Low cloud Point Biodiesel 9/4/2014 Low Cloud Point Biodiesel Shanti Christopher Johnson Mentors: Dr. David Hackleman Dr. Travis Walker School of Chemical, Biological, and Environmental Engineering Oregon State University, 103 Gleeson Hall Corvallis, OR 97331 BioResource Research, Bioenergy Oregon State University, 137 Strand Agriculture Hall Corvallis, OR 97331 1 Shanti Johnson Low cloud Point Biodiesel 9/4/2014 _________________________________________________ _______________ David Hackleman, June 3, 2014 Department of Chemical, Biological, & Environmental Engineering _________________________________________________ _______________ Travis Walker June 3, 2014 Department of Chemical, Biological, & Environmental Engineering _________________________________________________ ________________ Katharine G. Field, BRR Director June 3, 2014 ©Copyright by Shanti C. Johnson, June 3, 2014 I understand that by project will become part of the permanent collection for the Oregon State University Library, and will become part of the Scholars Archive collection for BioResources Research. My signature below authorizes release of my project and thesis to any reader upon request. ____________________________________________ ______________ Shanti C. Johnson June 3, 2014 2 Shanti Johnson Low cloud Point Biodiesel 9/4/2014 Abstract The synergetic interplay between the removal of saturated methyl esters and the addition of deoxygenated fatty acids on depressing the cloud point temperature of biodiesel was investigated in this study. Canola biodiesel was produced in batch as a standard with a cloud point of 1.37 +/- 6.3 oC. Saturated methyl esters were removed from the standard via urea fractionation resulting in a low saturate biodiesel with a cloud point of -21.06 +/- 0.5 oC. Heptadecene was used as a standard for the major products produced from the deoxygenation of saturated fatty acids. -

Lecture 9: Polymer Solutions – Phase Behavior
Prof. Tibbitt Lecture 9 Networks & Gels Lecture 9: Polymer solutions { Phase behavior Prof. Mark W. Tibbitt { ETH Z¨urich { 17 March 2020 1 Suggested reading • Molecular Driving Forces { Dill and Bromberg: Chapter 32 • Polymer Physics { Rubinstein and Colby: Chapters 4,5 • Soft Condensed Matter { Jones: Chapters 3,9 2 Flory-Huggins Theory In the last lecture, we derived the Flory-Huggins Theory for the Free Energy of mixing of polymer solutions. The derivation was general for species 1 and 2 with degree of polymerization x1 and x2 and is a more general expression of regular solution theory. In this discussion, we will look at what the Flory-Huggins theory tells us about phase behavior of polymers and how this differs from the behavior of small molecules. As we are no longer considering a lattice with N sites, we will revert back to using the term N as the degree of polymerization of our two species. Again we will consider two generic species A and B with degrees of polymerization NA and NB. Recall the form of the Free Energy of mixing per site from Flory-Huggins Theory: M ∆F M φA φB = ∆F¯ = ln φA + ln φB + χφAφB : (1) NkBT NA NB We will now use the term ∆F¯M as the Free Energy of mixing per site. 3 Phase behavior of polymer solutions In order to work with polymer solutions for industrial and biomedical applications, it is essential to understand the phase behavior of the systems. For example, we need to know under which conditions a polymer will dissolve in a solvent or if two polymers will be miscible.