PDF Download
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Breast Cancer Antiestrogen Resistance-3 Expression Regulates Breast Cancer Cell Migration Through Promotion of P130cas Membrane Localization Andmembrane Ruffling
Research Article Breast Cancer Antiestrogen Resistance-3 Expression Regulates Breast Cancer Cell Migration through Promotion of p130Cas Membrane Localization andMembrane Ruffling Randy S. Schrecengost,1 Rebecca B. Riggins,2 Keena S. Thomas,1 Michael S. Guerrero,1 and Amy H. Bouton1 1Department of Microbiology, University of Virginia Health System, Charlottesville, Virginia and 2Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, District of Columbia Abstract antiestrogen resistance-1 (BCAR1; also known as p130Cas; refs. 2, 3). Antiestrogens such as tamoxifen are widely used in the clinic A second protein, BCAR3 (the human homologue of the murine to treat estrogen receptor–positive breast tumors. Resistance protein AND-34), was identified in a genetic screen along with to tamoxifen can occur either de novo or develop over time in BCAR1 as a gene product whose overexpression conferred tamoxifen resistance in vitro (4). BCAR3 is a member of the novel a large proportion of these tumors. Additionally, resistance is S p associated with enhanced motility and invasiveness in vitro. rc homology 2 (SH2)–containing rotein (NSP) family that One molecule that has been implicated in tamoxifen resis- includes two other members, Chat/SHEP1 and NSP1. These tance, breast cancer antiestrogen resistance-3 (BCAR3), has proteins share a common domain structure consisting of an also been shown to regulate migration of fibroblasts. In this amino-terminal SH2 domain and a carboxyl-terminal domain with study, we investigated the role of BCAR3 in breast cancer cell sequence homology to the Cdc25-family of guanine nucleotide exchange factors (GEF). Several studies have shown that BCAR3 migration and invasion. -

Physiological Signaling and Structure of the HGF Receptor MET
Biomedicines 2015, 3, 1-31; doi:10.3390/biomedicines3010001 OPEN ACCESS biomedicines ISSN 2227-9059 www.mdpi.com/journal/biomedicines/ Review Physiological Signaling and Structure of the HGF Receptor MET Gianluca Baldanzi 1,* and Andrea Graziani 1,2 1 Department Translational Medicine, University Piemonte Orientale, via Solaroli 17, 28100 Novara, Italy 2 Università Vita-Salute San Raffaele, via Olgettina 58, 20132 Milano, Italy; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +39-0321-660527; Fax: +39-0321-620421. Academic Editor: Zimmer Yitzhak Received: 30 September 2014 / Accepted: 9 December 2014 / Published: 31 December 2014 Abstract: The “hepatocyte growth factor” also known as “scatter factor”, is a multifunctional cytokine with the peculiar ability of simultaneously triggering epithelial cell proliferation, movement and survival. The combination of those proprieties results in the induction of an epithelial to mesenchymal transition in target cells, fundamental for embryogenesis but also exploited by tumor cells during metastatization. The hepatocyte growth factor receptor, MET, is a proto-oncogene and a prototypical transmembrane tyrosine kinase receptor. Inhere we discuss the MET molecular structure and the hepatocyte growth factor driven physiological signaling which coordinates epithelial proliferation, motility and morphogenesis. Keywords: signaling pathways; tyrosine kinase receptor; protein–protein interaction; SH2 domain; post translational modification; signal transduction 1. Background Introduction The Hepatocyte Growth Factor (HGF) was originally identified as a soluble factor promoting hepatocyte growth and liver regeneration [1]. In a parallel way a Scatter Factor (SF) was identified as cytokine secreted by fibroblast promoting dissociation and motility of epithelial cells in culture [2]. -

The Extracellular Matrix for Bone Regeneration
This chapter is based on human mesenchymal stromal cells in adhesion to cell-derived 5 extracellular matrix and titanium: a comparative kinome profi le analysis Marta Baroncelli1^, Gwenny M. Fuhler2^, Jeroen van de Peppel1, Willian F. Zambuzzi3, Johannes P.T.M van Leeuwen1, Bram C.J van der Eerden1°, Maikel P. Peppelenbosch2° 1Department of Internal Medicine, Erasmus MC. University Medical Center Rotterdam, Wytemaweg 80, 3015 CN, Rotterdam, The Netherlands 2Department of Gastroenterology and Hepatology, Erasmus MC, University Medical Center Rotterdam, Wytemaweg 80, 3015 CN, Rotterdam, The Netherlands 3Laboratorio de Bioensaios e Dinâmica Celular, Dep.to de Quimica e Bioquimica, Instituto de Biociências, Universidade Estadual Paulista- UNESP, Campus Botucatu, São Paulo, SP Brazil. ^, °: both authors contributed equally to this work Submitted Chapter 5 ABSTRACT The extracellular matrix (ECM) is an essential component of tissue architecture that physically supports cells and actively influences stem cell behaviour, by modulating kinase-mediated signaling cascades that are the key regulators of signal transduc- tion. Cell-derived ECMs have recently emerged in the context of bone regeneration, as they reproduce physiological tissue-architecture and ameliorate the promising properties of mesenchymal stromal cells (MSCs). Titanium scaffolds show good mechanical properties and porosity to facilitate cell adhesion, and thus have been routinely used for bone tissue engineering applications. The aim of this study was to analyze the kinomic signature of human MSCs in adhesion to an osteoblast-derived ECM that we have previously shown to be osteopromotive, and to compare it to MSCs on titanium. PamChip kinase array analysis revealed 63 phosphorylated peptides on ECM and 59 on titanium, with MSCs on ECM exhibiting significant higher levels of kinase activity than those on titanium. -

Human Non-Small Cell Lung Cancer (H3255) Cell Line; Trypsin Digest; Antibody: Py; CST #9411 Treatments: Untreated (Heavy), Iressa-Treated (Light)
TABLE: TYROSINE PHOSPHOSCAN® FINAL RESULTS, SILAC StuDY DEsiGN: Human non-small cell lung cancer (H3255) cell line; Trypsin Digest; Antibody: pY; CST #9411 Treatments: Untreated (Heavy), Iressa-treated (Light) Fold-Change Count (Iressa / in Index Untreated ) Protein Name Phosphorylation Site Description Peptide Accession kD Study Actin binding proteins 2 -1.6 CTNNA1 177 Catenin alpha-1 (Cadherin-associated protein) NAGNEQDLGIQyK P35221 100 4 3 -4.5 CTNND1 248 Catenin delta-1 (Cadherin-associated Src substrate) (p120(cas)) YRPSMEGyR O60716 108 2 4 -3.9 CTNND1 865 Catenin delta-1 (Cadherin-associated Src substrate) (p120(cas)) SQSSHSyDDSTLPLIDR O60716 108 1 5 -5.5 CTNND1 904 Catenin delta-1 (Cadherin-associated Src substrate) (p120(cas)) SLDNNySTPNERGDHNR O60716 108 1 6 -3 CTNND1 904 Catenin delta-1 (Cadherin-associated Src substrate) (p120(cas)) SLDNNySTPNER O60716 108 4 7 -1.8 CTNND1 §228 Catenin delta-1 (Cadherin-associated Src substrate) (p120(cas)) HYEDGYPGGSDNyGsLSR O60716 108 4 8 -1.8 CTNND1 §228 Catenin delta-1 (Cadherin-associated Src substrate) (p120(cas)) HYEDGYPGGSDNyGSLSR O60716 108 6 9 -1.1 CTNND1 §257 Catenin delta-1 (Cadherin-associated Src substrate) (p120(cas)) QDVyGPQPQVR O60716 108 1 0 -6. CTNND1 §280 Catenin delta-1 (Cadherin-associated Src substrate) (p120(cas)) FHPEPyGLEDDQR O60716 108 3 -3 CTNND1 §96 Catenin delta-1 (Cadherin-associated Src substrate) (p120(cas)) LNGPQDHSHLLySTIPR O60716 108 1 2 -. DBNL §162 Drebrin-like protein (SH3 domain-containing protein 7) (HIP-55) FQDVGPQAPVGSVyQK Q9UJU6 48 2 3 Adaptor/scaffold -

Supplemental Data
Article TCF7L2 is a master regulator of insulin production and processing ZHOU, Yuedan, et al. Abstract Genome-wide association studies have revealed >60 loci associated with type 2 diabetes (T2D), but the underlying causal variants and functional mechanisms remain largely elusive. Although variants in TCF7L2 confer the strongest risk of T2D among common variants by presumed effects on islet function, the molecular mechanisms are not yet well understood. Using RNA-sequencing, we have identified a TCF7L2-regulated transcriptional network responsible for its effect on insulin secretion in rodent and human pancreatic islets. ISL1 is a primary target of TCF7L2 and regulates proinsulin production and processing via MAFA, PDX1, NKX6.1, PCSK1, PCSK2 and SLC30A8, thereby providing evidence for a coordinated regulation of insulin production and processing. The risk T-allele of rs7903146 was associated with increased TCF7L2 expression, and decreased insulin content and secretion. Using gene expression profiles of 66 human pancreatic islets donors', we also show that the identified TCF7L2-ISL1 transcriptional network is regulated in a genotype-dependent manner. Taken together, these results demonstrate that not only synthesis of [...] Reference ZHOU, Yuedan, et al. TCF7L2 is a master regulator of insulin production and processing. Human Molecular Genetics, 2014, vol. 23, no. 24, p. 6419-6431 DOI : 10.1093/hmg/ddu359 PMID : 25015099 Available at: http://archive-ouverte.unige.ch/unige:45177 Disclaimer: layout of this document may differ from the published -
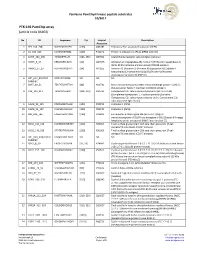
PTK-196 Pamchip Array (Article Code 86402)
PamGene PamChip4 kinase peptide substrates 10/2017 PTK-196 PamChip array (article code 86402) No ID Sequence Tyr Uniprot Description Accession 1 EFS_246_258 GGTDEGIYDVPLL [253] O43281 Embryonal Fyn-associated substrate (HEFS). 2 41_654_666 LDGENIYIRHSNL [660] P11171 Protein 4.1 (Band 4.1) (P4.1) (EPB4.1) (4.1R). 3 ACHD_383_395 YISKAEEYFLLKS [383, 390] Q07001 Acetylcholine receptor subunit delta precursor. 4 AMPE_5_17 EREGSKRYCIQTK [12] Q07075 Glutamyl aminopeptidase (EC 3.4.11.7) (EAP) (Aminopeptidase A) (APA) (Differentiation antigen gp160) (CD249 antigen). 5 ANXA2_17_29 HSTPPSAYGSVKA [24] P07355 Annexin A2 (Annexin-2) (Annexin II) (Lipocortin II) (Calpactin I heavychain) (Chromobindin-8) (p36) (Protein I) (Placental anticoagulantprotein IV) (PAP-IV). 6 ART_004_EAIYAAP EAIYAAPFAKKK NA NA NA FAKKKXC 7 B3AT_39_51 TEATATDYHTTSH [46] P02730 Band 3 anion transport protein (Anion exchange protein 1) (AE 1) (Solute carrier family 4 member 1) (CD233 antigen). 8 C1R_199_211 TEASGYISSLEYP [204, 210] P00736 Complement C1r subcomponent precursor (EC 3.4.21.41) (Complementcomponent 1, r subcomponent) [Contains: Complement C1r subcomponentheavy chain; Complement C1r subcomponent light chain]. 9 CALM_93_105 FDKDGNGYISAAE [100] P0DP23 Calmodulin (CaM). 10 CALM_95_107 KDGNGYISAAELR [100] P0DP23 Calmodulin (CaM). 11 CBL_693_705 EGEEDTEYMTPSS [700] P22681 E3 ubiquitin-protein ligase CBL (EC 6.3.2.-) (Signal transductionprotein CBL) (Proto-oncogene c-CBL) (Casitas B-lineage lymphoma proto-oncogene) (RING finger protein 55). 12 CD3Z_116_128 KDKMAEAYSEIGM [123] P20963 T-cell surface glycoprotein CD3 zeta chain precursor (T-cell receptorT3 zeta chain) (CD247 antigen). 13 CD3Z_146_158 STATKDTYDALHM [153] P20963 T-cell surface glycoprotein CD3 zeta chain precursor (T-cell receptorT3 zeta chain) (CD247 antigen). 14 ART_003_EAI(pY)AAP EAI(pY)AAPFAKKK NA NA NA FAKKKXC 15 CDK2_8_20 EKIGEGTYGVVYK [15, 19] P24941 Cyclin-dependent kinase 2 (EC:2.7.11.22) Cell division protein kinase 2 (EC 2.7.11.22) (p33 protein kinase). -

Somamer Reagents Generated to Human Proteins Number Somamer Seqid Analyte Name Uniprot ID 1 5227-60
SOMAmer Reagents Generated to Human Proteins The exact content of any pre-specified menu offered by SomaLogic may be altered on an ongoing basis, including the addition of SOMAmer reagents as they are created, and the removal of others if deemed necessary, as we continue to improve the performance of the SOMAscan assay. However, the client will know the exact content at the time of study contracting. SomaLogic reserves the right to alter the menu at any time in its sole discretion. Number SOMAmer SeqID Analyte Name UniProt ID 1 5227-60 [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial Q15118 2 14156-33 14-3-3 protein beta/alpha P31946 3 14157-21 14-3-3 protein epsilon P62258 P31946, P62258, P61981, Q04917, 4 4179-57 14-3-3 protein family P27348, P63104, P31947 5 4829-43 14-3-3 protein sigma P31947 6 7625-27 14-3-3 protein theta P27348 7 5858-6 14-3-3 protein zeta/delta P63104 8 4995-16 15-hydroxyprostaglandin dehydrogenase [NAD(+)] P15428 9 4563-61 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase gamma-1 P19174 10 10361-25 2'-5'-oligoadenylate synthase 1 P00973 11 3898-5 26S proteasome non-ATPase regulatory subunit 7 P51665 12 5230-99 3-hydroxy-3-methylglutaryl-coenzyme A reductase P04035 13 4217-49 3-hydroxyacyl-CoA dehydrogenase type-2 Q99714 14 5861-78 3-hydroxyanthranilate 3,4-dioxygenase P46952 15 4693-72 3-hydroxyisobutyrate dehydrogenase, mitochondrial P31937 16 4460-8 3-phosphoinositide-dependent protein kinase 1 O15530 17 5026-66 40S ribosomal protein S3 P23396 18 5484-63 40S ribosomal protein -

Dissecting Bcr-Abl Variant Signaling Pathways Using
DISSECTING BCR-ABL VARIANT SIGNALING PATHWAYS USING NOVEL INTERACTOME IDENTIFICATION STRATEGIES By Jevon A. Cutler A dissertation submitted to The Johns Hopkins University in conformity with the requirement of the degree of Doctor of Philosophy Baltimore, MD May 2018 © 2018 Jevon A. Cutler All Rights Reserved ABSTRACT Cell signaling is an essential function of cells and tissues. Understanding cell signaling necessitates technologies that can identify protein-protein interactions as well as post translational modifications to proteins within protein complexes. The goals of this study are (1) to understand how BCR-ABL variants differentially signal to produce different clinical/experimental phenotypes and (2) to develop novel interactome detection strategies to understand signaling. This dissertation describes an integrated approach of the use of proximity dependent labeling protein-protein interaction analysis assays coupled with global phosphorylation analysis to investigate the differences in signaling between two variants the oncogenic fusion protein, BCR-ABL. Two major types of leukemogenic BCR-ABL fusion proteins are p190BCR-ABL and p210BCR-ABL. Although the two fusion proteins are closely related, they can lead to different clinical outcomes. A thorough understanding of the signaling programs employed by these two fusion proteins is necessary to explain these clinical differences. Our findings suggest that p190BCR-ABL and p210BCR-ABL differentially activate important signaling pathways, such as JAK-STAT, and engage with molecules that indicate interaction with different subcellular compartments. In the case of p210BCR-ABL, we observed an increased engagement of molecules active proximal to the membrane and in the case of p190BCR-ABL, an engagement of molecules of the cytoskeleton. -

Supplementary Materials (PDF)
Proteomics of the mediodorsal thalamic nucleus in gastric ulcer induced by restraint-water-immersion-stress Sheng-Nan Gong, Jian-Ping Zhu, Ying-Jie Ma, Dong-Qin Zhao Table S1. The entire list of 2,853 proteins identified between the control and stressed groups Protein NO Protein name Gene name Accession No LogRatio 1 Tubulin alpha-1A chain Tuba1a TBA1A_RAT 0.2320 2 Spectrin alpha chain, non-erythrocytic 1 Sptan1 A0A0G2JZ69_RAT -0.0291 3 ATP synthase subunit alpha, mitochondrial Atp5f1a ATPA_RAT -0.1155 4 Tubulin beta-2B chain Tubb2b TBB2B_RAT 0.0072 5 Actin, cytoplasmic 2 Actg1 ACTG_RAT 0.0001 Sodium/potassium-transporting ATPase Atp1a2 6 subunit alpha-2 AT1A2_RAT -0.0716 7 Spectrin beta chain Sptbn1 A0A0G2K8W9_RAT -0.1158 8 Clathrin heavy chain 1 Cltc CLH1_RAT 0.0788 9 Dihydropyrimidinase-related protein 2 Dpysl2 DPYL2_RAT -0.0696 10 Glyceraldehyde-3-phosphate dehydrogenase Gapdh G3P_RAT -0.0687 Sodium/potassium-transporting ATPase Atp1a3 11 subunit alpha-3 AT1A3_RAT 0.0391 12 ATP synthase subunit beta, mitochondrial Atp5f1b ATPB_RAT 0.1772 13 Cytoplasmic dynein 1 heavy chain 1 Dync1h1 M0R9X8_RAT 0.0527 14 Myelin basic protein transcript variant N Mbp I7EFB0_RAT 0.0696 15 Microtubule-associated protein Map2 F1LNK0_RAT -0.1053 16 Pyruvate kinase PKM Pkm KPYM_RAT -0.2608 17 D3ZQQ5_RAT 0.0087 18 Plectin Plec F7F9U6_RAT -0.0076 19 14-3-3 protein zeta/delta Ywhaz A0A0G2JV65_RAT -0.2431 20 2',3'-cyclic-nucleotide 3'-phosphodiesterase Cnp CN37_RAT -0.0495 21 Creatine kinase B-type Ckb KCRB_RAT -0.0514 Voltage-dependent anion-selective channel -

Anti-Crkii Antibody (ARG54108)
Product datasheet [email protected] ARG54108 Package: 100 μl anti-CrkII antibody Store at: -20°C Summary Product Description Mouse Monoclonal antibody recognizes CRK Tested Reactivity Hu Tested Application WB Host Mouse Clonality Monoclonal Isotype IgG2b Target Name CrkII Antigen Species Human Immunogen Purified recombinant human CrkII protein fragments expressed in E.coli. Conjugation Un-conjugated Alternate Names CRKII; p38; Proto-oncogene c-Crk; Adapter molecule crk Application Instructions Application table Application Dilution WB 1:1000 Application Note * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. Calculated Mw 34 kDa Observed Size 34 kDa Properties Form Liquid Purification Affinity purified Buffer 0.1M Tris-Glycine (pH 7.4), 150 mM NaCl, 0.2% Sodium azide and 50% Glycerol Preservative 0.2% Sodium azide Stabilizer 50% Glycerol Concentration 1 mg/ml Storage instruction For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. Note For laboratory research only, not for drug, diagnostic or other use. www.arigobio.com 1/2 Bioinformation Database links GeneID: 1398 Human Swiss-port # P46108 Human Gene Symbol CRK Gene Full Name v-crk avian sarcoma virus CT10 oncogene homolog Background The Crk-I and Crk-II forms differ in their biological activities. Crk-II has less transforming activity than Crk- I. Crk-II mediates attachment-induced MAPK8 activation, membrane ruffling and cell motility in a Rac- dependent manner. -

Molecular Mechanisms Involved in Neural Substructure Development During Phosphodiesterase Inhibitor Treatment of Mesenchymal Stem Cells
International Journal of Molecular Sciences Article Molecular Mechanisms Involved in Neural Substructure Development during Phosphodiesterase Inhibitor Treatment of Mesenchymal Stem Cells Jerome Fajardo, Bruce K. Milthorpe and Jerran Santos * Advanced Tissue Engineering and Stem Cell Biology Group, School of Life Sciences, Faculty of Science, University of Technology Sydney, Sydney, NSW 2007, Australia; [email protected] (J.F.); [email protected] (B.K.M.) * Correspondence: [email protected] Received: 18 June 2020; Accepted: 6 July 2020; Published: 9 July 2020 Abstract: Stem cells are highly important in biology due to their unique innate ability to self-renew and differentiate into other specialised cells. In a neurological context, treating major injuries such as traumatic brain injury, spinal cord injury and stroke is a strong basis for research in this area. Mesenchymal stem cells (MSC) are a strong candidate because of their accessibility, compatibility if autologous, high yield and multipotency with a potential to generate neural cells. With the use of small-molecule chemicals, the neural induction of stem cells may occur within minutes or hours. Isobutylmethyl xanthine (IBMX) has been widely used in cocktails to induce neural differentiation. However, the key molecular mechanisms it instigates in the process are largely unknown. In this study we showed that IBMX-treated mesenchymal stem cells induced differentiation within 24 h with the unique expression of several key proteins such as Adapter protein crk, hypoxanthine-guanine phosphoribosyltransferase, DNA topoisomerase 2-beta and Cell division protein kinase 5 (CDK5), vital in linking signalling pathways. Furthermore, the increased expression of basic fibroblast growth factor in treated cells promotes phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase (MAPK) cascades and GTPase–Hras interactions. -

Molecular Mechanisms of Action of Imatinib Mesylate in Human Ovarian Cancer: a Proteomic Analysis BHAVINKUMAR B
CANCER GENOMICS & PROTEOMICS 5: 137-150 (2008) Molecular Mechanisms of Action of Imatinib Mesylate in Human Ovarian Cancer: A Proteomic Analysis BHAVINKUMAR B. PATEL 1, ΥΙΝ Α. HE 1, XIN-MING LI 1, ANDREY FROLOV 3, LISA VANDERVEER 2, CAROLYN SLATER 2, RUSSELL J. SCHILDER 2, MARGARET VON MEHREN 2, ANDREW K. GODWIN 2 and ANTHONY T. YEUNG 1 Division of 1Basic Science, and 2Medical Science, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, Pennsylvania; 3Department of Surgery, University of Alabama at Birmingham, 1824 6th Ave South, Birmingham, Alabama, U.S.A. Abstract. Background: Imatinib mesylate (Gleevec ®, Ovarian carcinoma is the fifth leading cause of cancer death Novartis, Basel, Switzerland) is a small-molecule tyrosine among women in the United States and the most common kinase inhibitor with activity against ABL, BCR-ABL, c-KIT, cause of death among gynecologic malignancies (1). and PDGFR α. Several clinical trials have evaluated the Ovarian cancer affects about 15 women for every 100,000 efficacy and safety of imatinib in patients with ovarian women under the age of 40 and over 50 women for every carcinoma who have persistent or recurrent disease following 100,000 women above the age of 70 (1). The five-year front-line platinum/taxane based chemotherapy. However, there survival rate for patients with advanced stage ovarian cancer is limited pre-clinical and clinical data on the molecular is only 29% which is in contrast to the women with tumors targets and action of imatinib in ovarian cancer. Materials and confined to the ovaries exceeding 90% (1). The cornerstone Methods: Human ovarian cancer cells (A2780) were treated of management for advanced ovarian cancer involves with imatinib mesylate for either 6 or 24 h.