Raphus Cucullatus): Application of a CT-Based Mass Estimation Technique
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
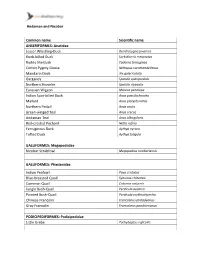
Andaman and Nicobar Common Name Scientific Name
Andaman and Nicobar Common name Scientific name ANSERIFORMES: Anatidae Lesser Whistling-Duck Dendrocygna javanica Knob-billed Duck Sarkidiornis melanotos Ruddy Shelduck Tadorna ferruginea Cotton Pygmy-Goose Nettapus coromandelianus Mandarin Duck Aix galericulata Garganey Spatula querquedula Northern Shoveler Spatula clypeata Eurasian Wigeon Mareca penelope Indian Spot-billed Duck Anas poecilorhyncha Mallard Anas platyrhynchos Northern Pintail Anas acuta Green-winged Teal Anas crecca Andaman Teal Anas albogularis Red-crested Pochard Netta rufina Ferruginous Duck Aythya nyroca Tufted Duck Aythya fuligula GALLIFORMES: Megapodiidae Nicobar Scrubfowl Megapodius nicobariensis GALLIFORMES: Phasianidae Indian Peafowl Pavo cristatus Blue-breasted Quail Synoicus chinensis Common Quail Coturnix coturnix Jungle Bush-Quail Perdicula asiatica Painted Bush-Quail Perdicula erythrorhyncha Chinese Francolin Francolinus pintadeanus Gray Francolin Francolinus pondicerianus PODICIPEDIFORMES: Podicipedidae Little Grebe Tachybaptus ruficollis Andaman and Nicobar COLUMBIFORMES: Columbidae Rock Pigeon Columba livia Andaman Wood-Pigeon Columba palumboides Eurasian Collared-Dove Streptopelia decaocto Red Collared-Dove Streptopelia tranquebarica Spotted Dove Streptopelia chinensis Laughing Dove Streptopelia senegalensis Andaman Cuckoo-Dove Macropygia rufipennis Asian Emerald Dove Chalcophaps indica Nicobar Pigeon Caloenas nicobarica Andaman Green-Pigeon Treron chloropterus Green Imperial-Pigeon Ducula aenea Nicobar Imperial-Pigeon Ducula nicobarica Pied Imperial-Pigeon -

(2004): Identification, Distribution, and Function of Gastroliths in Dinosaurs
IDENTIFICATION, DISTRIBUTION, AND FUNCTION OF GASTROLITHS IN DINOSAURS AND EXTANT BIRDS WITH EMPHASIS ON OSTRICHES (STRUTHIO CAMELUS) Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von Oliver Wings aus Sangerhausen Bonn 2004 Diplodocid sauropod accidentally ingesting gastroliths while feeding on a cycad. IDENTIFICATION, DISTRIBUTION, AND FUNCTION OF GASTROLITHS IN DINOSAURS AND EXTANT BIRDS WITH EMPHASIS ON OSTRICHES (STRUTHIO CAMELUS) Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von Oliver Wings aus Sangerhausen Bonn 2004 Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn 1. Referent: Privat-Dozent Dr. Martin Sander 2. Referent: Professor Dr. Jes Rust Tag der Promotion: 02.12.2004 Diese Dissertation ist auf dem Hochschulschriftenserver der ULB Bonn http://hss.ulb.uni- bonn.de/diss_online elektronisch publiziert This dissertation is published electronically on the ULB Bonn server for university publications: http://hss.ulb.uni-bonn.de/diss_online Dedicated to Claudia. Thanks for everything. French proverb: “Il a un estomac d’autuche!” (literally: He has the stomach of an ostrich!) means: He can tolerate everything! Geheimnisvoll am lichten Tag Läßt sich Natur des Schleiers nicht berauben, Und was sie deinem Geist -

Federal Register/Vol. 85, No. 74/Thursday, April 16, 2020/Notices
21262 Federal Register / Vol. 85, No. 74 / Thursday, April 16, 2020 / Notices acquisition were not included in the 5275 Leesburg Pike, Falls Church, VA Comment (1): We received one calculation for TDC, the TDC limit would not 22041–3803; (703) 358–2376. comment from the Western Energy have exceeded amongst other items. SUPPLEMENTARY INFORMATION: Alliance, which requested that we Contact: Robert E. Mulderig, Deputy include European starling (Sturnus Assistant Secretary, Office of Public Housing What is the purpose of this notice? vulgaris) and house sparrow (Passer Investments, Office of Public and Indian Housing, Department of Housing and Urban The purpose of this notice is to domesticus) on the list of bird species Development, 451 Seventh Street SW, Room provide the public an updated list of not protected by the MBTA. 4130, Washington, DC 20410, telephone (202) ‘‘all nonnative, human-introduced bird Response: The draft list of nonnative, 402–4780. species to which the Migratory Bird human-introduced species was [FR Doc. 2020–08052 Filed 4–15–20; 8:45 am]‘ Treaty Act (16 U.S.C. 703 et seq.) does restricted to species belonging to biological families of migratory birds BILLING CODE 4210–67–P not apply,’’ as described in the MBTRA of 2004 (Division E, Title I, Sec. 143 of covered under any of the migratory bird the Consolidated Appropriations Act, treaties with Great Britain (for Canada), Mexico, Russia, or Japan. We excluded DEPARTMENT OF THE INTERIOR 2005; Pub. L. 108–447). The MBTRA states that ‘‘[a]s necessary, the Secretary species not occurring in biological Fish and Wildlife Service may update and publish the list of families included in the treaties from species exempted from protection of the the draft list. -

Nicobar Pigeon Caloenas Nicobarica
Nicobar Pigeon Caloenas nicobarica Class: Aves Order: Columbiformes Family: Columbidae Characteristics: Also known as the hackled pigeon, vulturine pigeon and white-tailed pigeon, the nicobar pigeon is medium-sized pigeon with a grey chest and head, metallic back and wings and a white tail. Its long feathers trailing down from the neck give it its distinct look (Lincoln Park Zoo). Behavior: Nicobar pigeons are nomadic, commuting between islands around New Guinea is flocks of up to 85 birds. They will only roost and breed on islands with no humans (Who Zoo). Reproduction: Nicobar pigeon males may spend days courting a female but, if she accepts him in the end, it pays off as they mate for life. The male chooses the nest site and brings twigs and other plant material to the female who builds the Range & Habitat: nest. One egg is produced per clutch and they usually clutch twice per Forests on uninhabited islands year. Both parents incubate the egg which hatches after about 30 days. The chicks stays in the nest for about a month (Rosamond Gifford Zoo). Diet: Wild: Hard seeds, fruit, insects, corn Zoo: Fruits, vegetables, greens, pheasant grains Conservation: Lifespan: up to 15 years in Nicobar pigeon numbers are declining. They fall victim to the pet trade, captivity, 8-12 years in the wild. logging on islands, and are trapped for food. Special Adaptations: Have a very FYI: muscular gizzard that allows them Unique to pigeons, they drink by sticking their beak in the water but don’t to eat nuts with very hard shells. -

Demonstration of the Potential of Environmental DNA As A
www.nature.com/scientificreports OPEN Demonstration of the potential of environmental DNA as a tool for the detection of avian species Received: 16 October 2017 Masayuki Ushio 1,2, Koichi Murata3,4, Tetsuya Sado5, Isao Nishiumi6, Masamichi Takeshita7, Accepted: 1 March 2018 Wataru Iwasaki 7 & Masaki Miya 5 Published: xx xx xxxx Birds play unique functional roles in the maintenance of ecosystems, such as pollination and seed dispersal, and thus monitoring bird species diversity is a frst step towards avoiding undesirable consequences of anthropogenic impacts on bird communities. In the present study, we hypothesized that birds, regardless of their main habitats, must have frequent contact with water and that tissues that contain their DNA that persists in the environment (environmental DNA; eDNA) could be used to detect the presence of avian species. To this end, we applied a set of universal PCR primers (MiBird, a modifed version of fsh/mammal universal primers) for metabarcoding avian eDNA. We confrmed the versatility of MiBird primers by performing in silico analyses and by amplifying DNAs extracted from bird tissues. Analyses of water samples from zoo cages of birds with known species composition suggested that the use of MiBird primers combined with Illumina MiSeq could successfully detect avian species from water samples. Additionally, analysis of water samples collected from a natural pond detected fve avian species common to the sampling areas. The present fndings suggest that avian eDNA metabarcoding would be a complementary detection/identifcation tool in cases where visual census of bird species is difcult. Environmental DNA (eDNA) is genetic material that persists in an environment and is derived from organ- isms living there, and researchers have recently been using eDNA to detect the presence of macro-organisms, particularly those living in aquatic/semiaquatic ecosystems1–5. -

West New Britain Extension July 22–27, 2017
WEST NEW BRITAIN EXTENSION JULY 22 –27, 2017 White-mantled Kingfisher (Dion Hobcroft) LEADER: DION HOBCROFT LIST COMPILED BY: DION HOBCROFT VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM WEST NEW BRITAIN EXTENSION JULY 22 –27, 2017 By Dion Hobcroft A Purple-bellied Lory dines out in a Coconut flower. (Dion Hobcroft) Our tour got off to a shaky start with a cancelled flight from Port Moresby to Hoskins. Luckily, the superb Airways Hotel honored our rooms from the previous day that we had missed due to another cancelled flight. Love you Air Niugini! After settling in to the very comfortable Walindi Dive Resort, with its well-planned rooms, tasty meals, and excellent staff, we headed out for our first birding in New Britain. We met Joel, a local villager, who guided us up beyond his village into the forest. He led us to a New Britain Boobook, a small hawk-owl he keeps tabs on (and has done so for the past few years). With the elections on, I joked with Joel that we should call the owl “Prime Minister Pete.” We had actually seen the Prime Minister the day before. Victor Emanuel Nature Tours 2 West New Britain Extension, 2017 After great looks at “Pete” we slowly wandered to a viewing area over the forest that was heaving with birds —lots of Eclectus Parrots, our first Blue-eyed Cockatoos, dozens of Red-knobbed and Yellowish imperial-pigeons, Long-tailed Myna, Variable Goshawk, an all-white Pied Coucal, a single Channel-billed Cuckoo (quite rare on NB), the beautiful Purple-bellied Lory, the scarce Black-bellied Myzomela, and great views of the colorful Knob-billed Fruit-Dove. -

Palau Bird Survey Report 2020
Abundance of Birds in Palau based on Surveys in 2005 Final Report, November 2020 Eric A. VanderWerf1 and Erika Dittmar1 1 Pacific Rim Conservation, 3038 Oahu Avenue, Honolulu, Hawaii 96822 Prepared for the Belau National Museum, Box 666, Koror Palau 96940 Endemic birds of Palau, from top left: White-breasted Woodswallow, Palau Fantail, Palau Fruit- dove, Rusty-capped Kingfisher. Photos by Eric VanderWerf. 1 TABLE OF CONTENTS ACKNOWLEDGMENTS .............................................................................................................. 3 EXECUTIVE SUMMARY ............................................................................................................ 4 INTRODUCTION .......................................................................................................................... 5 METHODS ..................................................................................................................................... 6 Description of Study Area and Transect Locations ............................................................ 6 Data Collection ................................................................................................................... 7 Data Analysis ...................................................................................................................... 7 Limitations of the Survey.................................................................................................... 9 RESULTS .................................................................................................................................... -

Goura Victoria: COLUMBIDAE) in the RAINFORESTS of NORTHERN PAPUA, INDONESIA
THE IMPACT OF HUNTING ON VICTORIA CROWNED PIGEON (Goura victoria: COLUMBIDAE) IN THE RAINFORESTS OF NORTHERN PAPUA, INDONESIA Dissertation for the award of degree of “Doctor rerum naturalium” (Dr.rer.nat) within the doctoral program biology of the Georg-August University School of Science (GAUSS) Submitted by Henderina Josefina Keiluhu Born in Sumbawa Besar-West Nusa Tenggara, Indonesia Göttingen, 2013 Thesis Committee Prof. Dr. M. Mühlenberg Johann Friedrich Blumenbach Institute of Zoology and Anthropology Prof. Dr. R. Willmann Johann Friedrich Blumenbach Institute of Zoology and Anthropology Members of the Examination Board Reviewer: Prof. Dr. M. Mühlenberg Johann Friedrich Blumenbach Institute of Zoology and Anthropology Second Reviewer: Prof. Dr. R. Willmann Johann Friedrich Blumenbach Institute of Zoology and Anthropology Further members of the Examination Board Prof. Dr. C. Leuschner Albrecht von Haller Institute of Plant Sciences Prof. Dr. E. Bergmeier Albrecht von Haller Institute of Plant Sciences Prof. Dr. H. Behling Albrecht von Haller Institute of Plant Sciences PD. Dr. T. Hörnschemeyer Johann Friedrich Blumenbach Institute of Zoology and Anthropology Place and date of the oral examination: Computer Room, Department of Conservation Biology, Center for Nature Conservation, Bürgerstrasse 50, 37073 Goettingen; October 30th, 2013 at 11.15 pm ii Acknowledgements I am very grateful to my supervisor Prof. Dr. M. Mühlenberg, Department of Conservation Biology, Georg-August University of Goettingen for enhancement my concepts about nature conservation. I also thank Prof. Dr. R. Willmann for being my second supervisor, and to Dr. Richard Noske for the valuable tutorial during proposal writing. The Deutscher Akademischer Austausch Dienst (DAAD) contributed generous financial support for my study. -

Between Species: Choreographing Human And
BETWEEN SPECIES: CHOREOGRAPHING HUMAN AND NONHUMAN BODIES JONATHAN OSBORN A DISSERTATION SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY GRADUATE PROGRAM IN DANCE STUDIES YORK UNIVERSITY TORONTO, ONTARIO MAY, 2019 ã Jonathan Osborn, 2019 Abstract BETWEEN SPECIES: CHOREOGRAPHING HUMAN AND NONHUMAN BODIES is a dissertation project informed by practice-led and practice-based modes of engagement, which approaches the space of the zoo as a multispecies, choreographic, affective assemblage. Drawing from critical scholarship in dance literature, zoo studies, human-animal studies, posthuman philosophy, and experiential/somatic field studies, this work utilizes choreographic engagement, with the topography and inhabitants of the Toronto Zoo and the Berlin Zoologischer Garten, to investigate the potential for kinaesthetic exchanges between human and nonhuman subjects. In tracing these exchanges, BETWEEN SPECIES documents the creation of the zoomorphic choreographic works ARK and ARCHE and creatively mediates on: more-than-human choreography; the curatorial paradigms, embodied practices, and forms of zoological gardens; the staging of human and nonhuman bodies and bodies of knowledge; the resonances and dissonances between ethological research and dance ethnography; and, the anthropocentric constitution of the field of dance studies. ii Dedication Dedicated to the glowing memory of my nana, Patricia Maltby, who, through her relentless love and fervent belief in my potential, elegantly willed me into another phase of life, while she passed, with dignity and calm, into another realm of existence. iii Acknowledgements I would like to thank my phenomenal supervisor Dr. Barbara Sellers-Young and my amazing committee members Dr. -

Kea (Nestor Notabilis) Care Manual
Kea (Nestor notabilis) CARE MANUAL CREATED BY THE AZA Kea Species Survival Plan® Program IN ASSOCIATION WITH THE AZA Parrot Taxon Advisory Group Kea (Nestor notabilis) Care Manual Kea (Nestor notabilis) Care Manual Published by the Association of Zoos and Aquariums in collaboration with the AZA Animal Welfare Committee Formal Citation: AZA Kea Species Survival Plan (Nestor notabilis). (2020). Kea Care Manual. Silver Spring, MD: Association of Zoos and Aquariums. Original Completion Date: July 1, 2019 Kea (Nestor notabilis) Care Manual Coordinator: Kimberly Klosterman, Cincinnati Zoo & Botanical Garden, Senior Avian Keeper, Kea SSP Vice Coordinator Authors and Significant Contributors: Krista Adlehart CRM, Woodland Park Zoo, Animal Management Registrar Amanda Ardente NVM, PhD, Walt Disney World, University of Florida, Nutrition Fellow Jackie Bray, MA Zoology CPBT-KA, Raptor Incorporated, Associate Director Cassandre Crawford MM, Northwest Local School District, Orchestra Director, Kea SSP Volunteer Thea Etchells, Denver Zoo, Bird Keeper Linda Henry, Board Member of Zoological Lighting Institute, SeaWorld San Diego Phillip Horvey, Sedgwick County Zoo, Senior Zookeeper, Masked Lapwing SSP Coordinator and Studbook Keeper Cari Inserra, San Diego Zoo, Lead Animal Trainer Kimberly Klosterman, Cincinnati Zoo & Botanical Garden, Senior Avian Keeper, Kea Care Manual Coordinator, Vice Coordinator Kea SSP Program Jessica Meehan, Denver Zoo, Bird Keeper, Kea SSP Coordinator and Studbook Keeper Jennifer Nollman DVM, Cincinnati Zoo & Botanical Garden, Associate Veterinarian Catherine Vine, Philadelphia Zoo, Avian Keeper Reviewers: Raoul Schwing PhD, Head of Kea Lab & Infrastructure Project Manager, Messerli Research Institute, University of Vienna, AU Tamsin Orr-Walker, BAAT, Co-founder, Trustee & Chair of Kea Conservation Trust, South Island Community Engagement Coordinator, NZ Nigel Simpson, EAZA Kea EEP Coordinator, Head of Operations, Wild Place Project, Bristol Zoological Society, UK Dr.rer.nat Gyula K. -

Birds at Woodland Park Zoo Pre-Visit Information for Teachers
BIRDS AT WOODLAND PARK ZOO PRE-VISIT INFORMATION FOR TEACHERS If you are planning a zoo field trip and wish to have your students focus on birds during their visit, this pre-visit sheet can help them get the most out of their time at the zoo. We have put together an overview of key concepts related to birds, a list of basic vocabulary words, and a checklist of bird species at Woodland Park Zoo. Knowledge and understanding of these main ideas will enhance your students’ zoo visit. OVERVIEW: There are over 10,000 species of birds currently identified worldwide, inhabiting a number of different biomes and exhibiting a range of adaptations. Woodland Park Zoo exhibits a wide variety of bird species (see attached checklist) in several different areas of the zoo. A bird field trip to the zoo could focus on the characteristics of birds (see “Concepts” below), comparing/contrasting different birds or learning about biomes and observing the physical characteristics of birds in different biomes. CONCEPTS: Birds share the following physical characteristics: Feathers Endothermic (warm-blooded) Eggs with shell and yolk Lack teeth, but have bony beaks Lightweight skeleton, bones with air spaces Good vision Adaptations for flight: Low body weight Streamlined form Efficient metabolism Specialized respiration and circulation Birds, like all plants and animals, have five basic needs to survive—food, water, shelter, air and space. They inhabit every continent on the planet and range in size from the bee hummingbird at 0.05 ounces (1.6 grams) to the North African ostrich at 275 pounds (125 kilograms). -

Conservation Considerations for Crowned Pigeons, Genus Goura
ORYX VOL 28 NO 1 JANUARY 1994 Conservation considerations for crowned pigeons, genus Goura Catherine E. King and Joeke Nijboer The three species of crowned pigeons are endemic to New Guinea and nearby islands where they are declining in numbers, especially near human settlements where hunting pressure is high. Little is known about their biology and ecology or about the magnitude of the impact of the threats from hunting, capture for trade, and loss of their lowland forest habitat. The authors make recommendations for action needed to secure the future for these large and attractive birds. Introduction and flooded forests from sea level to 500 m; G. s. scheepmakeri occurs in south-eastern Papua The genus Goura, comprising three crowned New Guinea from Hall Sound to Orangerie pigeon species, can be considered 'one of the Bay and Goura s. sclateri in southern Irian Jaya most remarkable evolutionary products of the and Papua New Guinea between the Mimika long isolation of New Guinea's tropical humid and Fly Rivers (Rand and Gillard, 1968; forest' (Beehler, 1991). Despite their large size Goodwin, 1983; Beehler et al., 1986). The range and their widespread distribution within New has not been clearly defined but apparently Guinea and nearby islands, to which they are suitable habitat extends along the southern endemic, very little is known about their bi- coast from Etna Bay to Orangerie Bay ology and status in the wild (Beehler, 1991). Goura victoria, the Victoria crowned pigeon, The purpose of this article is to summarize inhabits flat, lowland and partly inundated what information is available concerning this tropical forests near sea level.