Analyzing the Complex Machinery of Cell Wall Biosynthesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Yeast Genome Gazetteer P35-65
gazetteer Metabolism 35 tRNA modification mitochondrial transport amino-acid metabolism other tRNA-transcription activities vesicular transport (Golgi network, etc.) nitrogen and sulphur metabolism mRNA synthesis peroxisomal transport nucleotide metabolism mRNA processing (splicing) vacuolar transport phosphate metabolism mRNA processing (5’-end, 3’-end processing extracellular transport carbohydrate metabolism and mRNA degradation) cellular import lipid, fatty-acid and sterol metabolism other mRNA-transcription activities other intracellular-transport activities biosynthesis of vitamins, cofactors and RNA transport prosthetic groups other transcription activities Cellular organization and biogenesis 54 ionic homeostasis organization and biogenesis of cell wall and Protein synthesis 48 plasma membrane Energy 40 ribosomal proteins organization and biogenesis of glycolysis translation (initiation,elongation and cytoskeleton gluconeogenesis termination) organization and biogenesis of endoplasmic pentose-phosphate pathway translational control reticulum and Golgi tricarboxylic-acid pathway tRNA synthetases organization and biogenesis of chromosome respiration other protein-synthesis activities structure fermentation mitochondrial organization and biogenesis metabolism of energy reserves (glycogen Protein destination 49 peroxisomal organization and biogenesis and trehalose) protein folding and stabilization endosomal organization and biogenesis other energy-generation activities protein targeting, sorting and translocation vacuolar and lysosomal -

Open Matthew R Moreau Ph.D. Dissertation Finalfinal.Pdf
The Pennsylvania State University The Graduate School Department of Veterinary and Biomedical Sciences Pathobiology Program PATHOGENOMICS AND SOURCE DYNAMICS OF SALMONELLA ENTERICA SEROVAR ENTERITIDIS A Dissertation in Pathobiology by Matthew Raymond Moreau 2015 Matthew R. Moreau Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy May 2015 The Dissertation of Matthew R. Moreau was reviewed and approved* by the following: Subhashinie Kariyawasam Associate Professor, Veterinary and Biomedical Sciences Dissertation Adviser Co-Chair of Committee Bhushan M. Jayarao Professor, Veterinary and Biomedical Sciences Dissertation Adviser Co-Chair of Committee Mary J. Kennett Professor, Veterinary and Biomedical Sciences Vijay Kumar Assistant Professor, Department of Nutritional Sciences Anthony Schmitt Associate Professor, Veterinary and Biomedical Sciences Head of the Pathobiology Graduate Program *Signatures are on file in the Graduate School iii ABSTRACT Salmonella enterica serovar Enteritidis (SE) is one of the most frequent common causes of morbidity and mortality in humans due to consumption of contaminated eggs and egg products. The association between egg contamination and foodborne outbreaks of SE suggests egg derived SE might be more adept to cause human illness than SE from other sources. Therefore, there is a need to understand the molecular mechanisms underlying the ability of egg- derived SE to colonize the chicken intestinal and reproductive tracts and cause disease in the human host. To this end, the present study was carried out in three objectives. The first objective was to sequence two egg-derived SE isolates belonging to the PFGE type JEGX01.0004 to identify the genes that might be involved in SE colonization and/or pathogenesis. -

Fucosyltransferase 8 As a Functional Regulator of Nonsmall Cell Lung Cancer
Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer Chien-Yu Chena,b, Yi-Hua Janb, Yi-Hsiu Juanc, Chih-Jen Yangd, Ming-Shyan Huangd, Chong-Jen Yuc, Pan-Chyr Yangc, Michael Hsiaob, Tsui-Ling Hsub,1, and Chi-Huey Wongb,1 aInstitute of Biochemical Sciences, National Taiwan University, Taipei 106, Taiwan; bGenomics Research Center, Academia Sinica, Taipei 115, Taiwan; cDepartment of Internal Medicine, National Taiwan University Hospital, Taipei 100, Taiwan; and dDepartment of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung 807, Taiwan Contributed by Chi-Huey Wong, November 26, 2012 (sent for review August 17, 2012) The up-regulation of fucosyltransferase 8 (FUT8), the only enzyme downstream signaling. Furthermore, the increase in core fuco- catalyzing α1,6-fucosylation in mammals, has been observed in sylation on E-cadherin has been shown to strengthen cell–cell several malignant cancers including liver, ovarian, thyroid, and co- adhesion (10). lorectal cancers. However, the pathological role and the regulatory Both transgenic and knockout mouse models have been gen- mechanism of FUT8 in cancers remain largely unknown. In the cur- erated to study the physiological role of FUT8 (7, 11, 12). Ec- rent study, we report that the expression of FUT8 is up-regulated in topic expression of FUT8 in mice results in an accumulation of nonsmall cell lung cancer (NSCLC) and correlates with tumor me- lipid droplets in hepatocytes and proximal renal tubular cells. tastasis, disease recurrence, and poor survival in patients with This steatosis-like phenotype observed in transgenic mice is NSCLC. Knocking down FUT8 in aggressive lung cancer cell lines linked to the activity of liver lysosomal acid lipase, which becomes significantly inhibits their malignant behaviors including in vitro inactive when over core-fucosylated (11), suggesting that excess invasion and cell proliferation, as well as in vivo metastasis and core fucosylation may lead to a breakdown of normal lipid me- tumor growth. -

Estudi Cinètic De L'activitat Enzimàtica De GT-MG517
PART EXPERIMENTAL - Materials i mètodes – - 229 - - 230 - - 231 - - 232 - Capítol 8: Protocols de biologia molecular - 233 - - 234 - Capítol 8: Protocols de biologia molecular 8 Protocols de biologia molecular 8.1 Capítol 2: Estudi de l’essencialitat de les possibles glicosiltransferases de Mycoplasma genitalium 8.1.1 Preparació de medi SP4 La preparació de medi SP4, medi complex que s’usa per al creixement de Mycoplasma genitalium, té lloc com s’explica a continuació. A la Taula 8.1 se’n presenten els components i els volums corresponents per a la preparació de 500 mL de medi líquid. Taula 8.1. Composició i preparació del medi SP4. Quantitat per Compost a 500 mL Preparació de la base PPLO (Difco) 1.75 g Triptona (Difco) 5 g Bactopeptona (Difco) 2.65 g Glucosa (Sigma-Aldrich) 2.5 g Aigua destil·lada Fins a 312 mL Preparació dels complements Yestolate 2 % autoclavat (Difco) 50 mL Roig de fenol 0.1 % pH 7 autoclavat 7 mL Extracte de llevat fresc 25 % 17.5 mL CMRL 10x (Invitrogen) 25 mL Sèrum boví fetal (Invitrogen) 85 mL Glutamina 29.2 mg/mL 1.71 mL Inicialment es mesclen tots els components corresponents a la base del medi i s’ajusta el pH a 7.8 amb NaOH 1 M. El conjunt s’autoclava 15 min a 120 ºC i es deixa refredar abans d’afegir-hi els complements. Un cop aquests s’han addicionat, cal comprovar que el pH del medi es manté entre 7.6 i 7.8. La preparació de l’extracte de llevat té lloc a partir de la dilució de 250 g de llevat fresc en 1 L d’aigua destil·lada. -

Comparative Analysis of High-Throughput Assays of Family-1 Plant Glycosyltransferases
International Journal of Molecular Sciences Article Comparative Analysis of High-Throughput Assays of Family-1 Plant Glycosyltransferases Kate McGraphery and Wilfried Schwab * Biotechnology of Natural Products, Technische Universität München, 85354 Freising, Germany; [email protected] * Correspondence: [email protected]; Tel.: +49-8161-712-912; Fax: +49-8161-712-950 Received: 27 January 2020; Accepted: 21 March 2020; Published: 23 March 2020 Abstract: The ability of glycosyltransferases (GTs) to reduce volatility, increase solubility, and thus alter the bioavailability of small molecules through glycosylation has attracted immense attention in pharmaceutical, nutraceutical, and cosmeceutical industries. The lack of GTs known and the scarcity of high-throughput (HTP) available methods, hinders the extrapolation of further novel applications. In this study, the applicability of new GT-assays suitable for HTP screening was tested and compared with regard to harmlessness, robustness, cost-effectiveness and reproducibility. The UDP-Glo GT-assay, Phosphate GT Activity assay, pH-sensitive GT-assay, and UDP2-TR-FRET assay were applied and tailored to plant UDP GTs (UGTs). Vitis vinifera (UGT72B27) GT was subjected to glycosylation reaction with various phenolics. Substrate screening and kinetic parameters were evaluated. The pH-sensitive assay and the UDP2-TR-FRET assay were incomparable and unsuitable for HTP plant GT-1 family UGT screening. Furthermore, the UDP-Glo GT-assay and the Phosphate GT Activity assay yielded closely similar and reproducible KM, vmax, and kcat values. Therefore, with the easy experimental set-up and rapid readout, the two assays are suitable for HTP screening and quantitative kinetic analysis of plant UGTs. This research sheds light on new and emerging HTP assays, which will allow for analysis of novel family-1 plant GTs and will uncover further applications. -
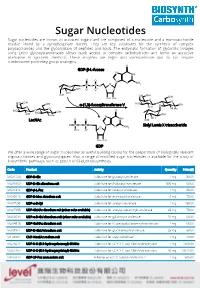
Sugar Nucleotides Sugar Nucleotides Are Known As Activated Sugars and Are Composed of a Nucleoside and a Monosaccharide Residue Linked by a Pyrophosphate Moiety
Sugar Nucleotides Sugar nucleotides are known as activated sugars and are composed of a nucleoside and a monosaccharide residue linked by a pyrophosphate moiety. They are key substrates for the synthesis of complex polysaccharides and the glycosylation of peptides and lipids. The enzymatic formation of glycosidic linkages using Leloir glycosyltransferases allows quick access to complex carbohydrates and forms an attractive alternative to synthetic methods. These enzymes are regio- and stereoselective and do not require cumbersome protecting group strategies. GDP-β-L-fucose α-(1,3)-fucosyltransferase V LacNAc Sialyl Lewis X trisaccharide GDP We offer a wide range of sugar nucleotides as useful building blocks for the preparation of biologically relevant oligosaccharides and glycoconjugates. Also, a range of modified sugar nucleotides is available for the study of biosynthetic pathways, such as Lipid A or (Sia)LeX biosynthesis. Code Product Activity Quantity Price ($) MG31129 GDP-D-Glc Substrate for glucosyltransferase 1 mg 50.00 MU08960 UDP-D-Glc disodium salt Substrate for β-glucosyltransferase 500 mg 60.00 MG01912 GDP-β-L-Fuc Substrate for fucosyltransferase 1 mg 85.00 MG05610 GDP-D-Man disodium salt Substrate for mannosyltransferase 5 mg 75.00 MU07658 UDP-α-D-Xyl Substrate for xylosyltransferase 1 mg 150.00 MU07955 UDP-GlcNAc disodium salt (other salts available) Substrate for acetylglucosaminyltransferase 25 mg 75.00 MU06699 UDP-α-D-Gal disodium salt (other salts available) Substrate for galactosyltransferase 10 mg 68.30 MU04515 UDP-GalNAc -

Supplementary Information
Supplementary information (a) (b) Figure S1. Resistant (a) and sensitive (b) gene scores plotted against subsystems involved in cell regulation. The small circles represent the individual hits and the large circles represent the mean of each subsystem. Each individual score signifies the mean of 12 trials – three biological and four technical. The p-value was calculated as a two-tailed t-test and significance was determined using the Benjamini-Hochberg procedure; false discovery rate was selected to be 0.1. Plots constructed using Pathway Tools, Omics Dashboard. Figure S2. Connectivity map displaying the predicted functional associations between the silver-resistant gene hits; disconnected gene hits not shown. The thicknesses of the lines indicate the degree of confidence prediction for the given interaction, based on fusion, co-occurrence, experimental and co-expression data. Figure produced using STRING (version 10.5) and a medium confidence score (approximate probability) of 0.4. Figure S3. Connectivity map displaying the predicted functional associations between the silver-sensitive gene hits; disconnected gene hits not shown. The thicknesses of the lines indicate the degree of confidence prediction for the given interaction, based on fusion, co-occurrence, experimental and co-expression data. Figure produced using STRING (version 10.5) and a medium confidence score (approximate probability) of 0.4. Figure S4. Metabolic overview of the pathways in Escherichia coli. The pathways involved in silver-resistance are coloured according to respective normalized score. Each individual score represents the mean of 12 trials – three biological and four technical. Amino acid – upward pointing triangle, carbohydrate – square, proteins – diamond, purines – vertical ellipse, cofactor – downward pointing triangle, tRNA – tee, and other – circle. -
Exploring the Edible Gum (Galactomannan) Biosynthesis and Its Regulation During Pod Developmental Stages in Clusterbean Using Co
www.nature.com/scientificreports OPEN Exploring the edible gum (galactomannan) biosynthesis and its regulation during pod developmental stages in clusterbean using comparative transcriptomic approach Sandhya Sharma1, Anshika Tyagi1, Harsha Srivastava1, G. Ramakrishna1, Priya Sharma1, Amitha Mithra Sevanthi1, Amolkumar U. Solanke1, Ramavtar Sharma2, Nagendra Kumar Singh1, Tilak Raj Sharma1,3 & Kishor Gaikwad1* Galactomannan is a polymer of high economic importance and is extracted from the seed endosperm of clusterbean (C. tetragonoloba). In the present study, we worked to reveal the stage-specifc galactomannan biosynthesis and its regulation in clusterbean. Combined electron microscopy and biochemical analysis revealed high protein and gum content in RGC-936, while high oil bodies and low gum content in M-83. A comparative transcriptome study was performed between RGC-936 (high gum) and M-83 (low gum) varieties at three developmental stages viz. 25, 39, and 50 days after fowering (DAF). Total 209,525, 375,595 and 255,401 unigenes were found at 25, 39 and 50 DAF respectively. Diferentially expressed genes (DEGs) analysis indicated a total of 5147 shared unigenes between the two genotypes. Overall expression levels of transcripts at 39DAF were higher than 50DAF and 25DAF. Besides, 691 (RGC-936) and 188 (M-83) candidate unigenes that encode for enzymes involved in the biosynthesis of galactomannan were identifed and analyzed, and 15 key enzyme genes were experimentally validated by quantitative Real-Time PCR. Transcription factor (TF) WRKY was observed to be co-expressed with key genes of galactomannan biosynthesis at 39DAF. We conclude that WRKY might be a potential biotechnological target (subject to functional validation) for developing high gum content varieties. -

The Metabolic Building Blocks of a Minimal Cell Supplementary
The metabolic building blocks of a minimal cell Mariana Reyes-Prieto, Rosario Gil, Mercè Llabrés, Pere Palmer and Andrés Moya Supplementary material. Table S1. List of enzymes and reactions modified from Gabaldon et. al. (2007). n.i.: non identified. E.C. Name Reaction Gil et. al. 2004 Glass et. al. 2006 number 2.7.1.69 phosphotransferase system glc + pep → g6p + pyr PTS MG041, 069, 429 5.3.1.9 glucose-6-phosphate isomerase g6p ↔ f6p PGI MG111 2.7.1.11 6-phosphofructokinase f6p + atp → fbp + adp PFK MG215 4.1.2.13 fructose-1,6-bisphosphate aldolase fbp ↔ gdp + dhp FBA MG023 5.3.1.1 triose-phosphate isomerase gdp ↔ dhp TPI MG431 glyceraldehyde-3-phosphate gdp + nad + p ↔ bpg + 1.2.1.12 GAP MG301 dehydrogenase nadh 2.7.2.3 phosphoglycerate kinase bpg + adp ↔ 3pg + atp PGK MG300 5.4.2.1 phosphoglycerate mutase 3pg ↔ 2pg GPM MG430 4.2.1.11 enolase 2pg ↔ pep ENO MG407 2.7.1.40 pyruvate kinase pep + adp → pyr + atp PYK MG216 1.1.1.27 lactate dehydrogenase pyr + nadh ↔ lac + nad LDH MG460 1.1.1.94 sn-glycerol-3-phosphate dehydrogenase dhp + nadh → g3p + nad GPS n.i. 2.3.1.15 sn-glycerol-3-phosphate acyltransferase g3p + pal → mag PLSb n.i. 2.3.1.51 1-acyl-sn-glycerol-3-phosphate mag + pal → dag PLSc MG212 acyltransferase 2.7.7.41 phosphatidate cytidyltransferase dag + ctp → cdp-dag + pp CDS MG437 cdp-dag + ser → pser + 2.7.8.8 phosphatidylserine synthase PSS n.i. cmp 4.1.1.65 phosphatidylserine decarboxylase pser → peta PSD n.i. -

Title Diurnal Metabolic Regulation of Isoflavones and Soyasaponins In
Diurnal metabolic regulation of isoflavones and soyasaponins Title in soybean roots Matsuda, Hinako; Nakayasu, Masaru; Aoki, Yuichi; Yamazaki, Author(s) Shinichi; Nagano, Atsushi J.; Yazaki, Kazufumi; Sugiyama, Akifumi Citation Plant direct (2020), 4(11) Issue Date 2020-11 URL http://hdl.handle.net/2433/259809 © 2020 The Authors. Plant Direct published by American Society of Plant Biologists and the Society for Experimental Biology and John Wiley & Sons Ltd; This is an open access article under the terms of the Creative Commons Attribution‐ Right NonCommercial‐NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non‐commercial and no modifications or adaptations are made. Type Journal Article Textversion publisher Kyoto University Received: 23 April 2020 | Revised: 23 September 2020 | Accepted: 14 October 2020 DOI: 10.1002/pld3.286 ORIGINAL RESEARCH Diurnal metabolic regulation of isoflavones and soyasaponins in soybean roots Hinako Matsuda1 | Masaru Nakayasu1 | Yuichi Aoki2 | Shinichi Yamazaki2 | Atsushi J. Nagano3 | Kazufumi Yazaki1 | Akifumi Sugiyama1 1Research Institute for Sustainable Humanosphere, Kyoto University, Gokasho, Abstract Uji, Japan Isoflavones and soyasaponins are major specialized metabolites accumulated in soy- 2 Tohoku Medical Megabank Organization, bean roots and secreted into the rhizosphere. Unlike the biosynthetic pathway, the Tohoku University, Sendai, Japan 3Faculty of Agriculture, Ryukoku University, transporters involved in metabolite -

Application of Human Glycosyltransferases in N-Glycan Synthesis and Their Substrate Specificity Studies
Georgia State University ScholarWorks @ Georgia State University Chemistry Dissertations Department of Chemistry 12-15-2016 Application of Human Glycosyltransferases in N-glycan Synthesis and Their Substrate Specificity Studies Angie Dayan Calderon Molina Georgia State University Follow this and additional works at: https://scholarworks.gsu.edu/chemistry_diss Recommended Citation Calderon Molina, Angie Dayan, "Application of Human Glycosyltransferases in N-glycan Synthesis and Their Substrate Specificity Studies." Dissertation, Georgia State University, 2016. https://scholarworks.gsu.edu/chemistry_diss/128 This Dissertation is brought to you for free and open access by the Department of Chemistry at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Chemistry Dissertations by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. APPLICATION OF HUMAN GLYCOSYLTRANSFERASES IN N-GLYCAN SYNTHESIS AND THEIR SUBSTRATE SPECIFICITY STUDIES by ANGIE DAYAN CALDERON MOLINA Under the Direction of Peng G. Wang, PhD ABSTRACT Glycoscience is important in many areas such as human health, energy and material science. Glycans have been shown to be involved in the pathophysiology of almost every major disease. Additional glycan structure knowledge is required to help advance personal medicine, and pharmaceutical developments, among others. For glycoscience to advance there is a need for large quantities of well-defined glycans and have quick access to glycosyltransferases for manipulating glycan synthesis. Herein, we will cover our efforts on studying the substrate specificities of human glycosyltransferases such as FUT8 and Gn-T V, and their application on N-glycan synthesis. Complex asymmetric N-glycan isomer structures have been related to many diseases such as breast cancer, among others. -

Advances in Understanding Glycosyltransferases from A
Available online at www.sciencedirect.com ScienceDirect Advances in understanding glycosyltransferases from a structural perspective Tracey M Gloster Glycosyltransferases (GTs), the enzymes that catalyse commonly activated nucleotide sugars, but can also be glycosidic bond formation, create a diverse range of lipid phosphates and unsubstituted phosphate. saccharides and glycoconjugates in nature. Understanding GTs at the molecular level, through structural and kinetic GTs have been classified by sequence homology into studies, is important for gaining insights into their function. In 96 families in the Carbohydrate Active enZyme data- addition, this understanding can help identify those enzymes base (CAZy) [1 ]. The CAZy database provides a highly which are involved in diseases, or that could be engineered to powerful predictive tool, as the structural fold and synthesize biologically or medically relevant molecules. This mechanism of action are invariant in most of the review describes how structural data, obtained in the last 3–4 families. Therefore, where the structure and mechanism years, have contributed to our understanding of the of a GT member for a given family has been reported, mechanisms of action and specificity of GTs. Particular some assumptions about other members of the family highlights include the structure of a bacterial can be made. Substrate specificity, however, is more oligosaccharyltransferase, which provides insights into difficult to predict, and requires experimental charac- N-linked glycosylation, the structure of the human O-GlcNAc terization of individual GTs. Determining both the transferase, and the structure of a bacterial integral membrane sugar donor and acceptor for a GT of unknown function protein complex that catalyses the synthesis of cellulose, the can be challenging, and is one of the reasons there are most abundant organic molecule in the biosphere.