Responses of Phytoplankton Benthic Propagules to Macronutrient Enrichment and Varying Light Intensities: Elucidation from Monsoonal Estuaries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Official Gazette
, ' ) 'REGD. GOA ~ 5 , . Panaji, 18th April, 1985 (Chaifra 28, 19C7J SERIES ,m No.3 OFFICIAL GAZETTE GOVERNMENT OF ,GOA~. DAMAN AND DIU- !,w .• 'GOVERNMENT OF GOA. DAMAN '0. I. D,Bran~)i; Pannjl i!ifOn)led that Shri I),Souza left Goa ""d lrtlo/~t9r Jew df'ys at Bombil,y tJiom., wItere,'?,!}!;, went to J!;qV.;ii.!t wllere lIe Is serving. Howevei, his0d4rel!S at AND DIU Kuw>dt Is .tlot !=)IYJl. " Department of Personnel and Administrative Reforms" !,I!avft ~~IIY ~.ed tM Pl"Ol! and cons Qf' the case ,0lIII )lave come to tile conclUSion 'tlmt Shri D'Souza, !:.ower General Adminlslralion and Co-ordination Division Dlvislon Clerk, W6rk1ng I!'the Office of the DePl!ty Colleetor, Office of the Collector of G:oa South, MargaI' under this Collectorate of Goa, by hl~ un authorised a~ee ft""rn duties and nof keepln~ the Office infO~ of his 'whereabouts has committed gross mis.:conduct Order and has, therefQre faUed to lnstntaln absolute' devotion to , duties. I, am there.fore, satisfied that Shri D'Souza, L. D. C .. ' No.'12(6)/128j83-EST/COr. iB pot a fit pe~ t~()r retention in Govei'IlJnent Service as' Shri Joseph D'Souza,' Lower Division Clerk 'Working in r.. D. C. I" am also Satisfied that it is nQtreasonablyprscU" the Office of the Deputy Collector, South, ~'\9 \!Ader , callie to !lold an Inqun-y in a, manner provided under Rule 14 this ColIectorate Is absent from duties from 8-12-1983. and 15 of the Central Civil Services (Classification, Control ,and Appeal) Rules, 1965 against Shri D'Souza.' Therefore, Shri Joseph D'Souza had applied for earned leave on I feel 'that ,the only course left to dispose of the case is 31-10-1983 for a period of 30 days with effect from 8-11·1983 by invocation of the provisions of ,Rule 19(ii) Ibid, ' to 7-12-1983 wbk:h was granted to him vl(j@ or4<!t' N(>. -

District Census Handbook, North Goa
CENSUS OF INDIA 1991 SERIES 6 GOA DISTRICT CENSUS HAND BOOK PART XII-A AND XII-B VILLAGE AND TOWN DIRECTORY AND VILLAGE AND TOWNWISE PRIMARY CENSUS ABSTRACT NORTH GOA DISTRICT S. RAJENDRAN DIRECTOR OF CENSUS OPERATIONS, GOA 1991 CENSUS PUBLICATIONS OF GOA ( All the Census Publications of this State will bear Series No.6) Central Government Publications Part Administration Report. Part I-A Administration Report-Enumeration. (For Official use only). Part I-B Administration Report-Tabulation. Part II General Population Tables Part II-A General Population Tables-A- Series. Part II-B Primary Census Abstract. Part III General Economic Tables Part III-A B-Series tables '(B-1 to B-5, B-l0, B-II, B-13 to B -18 and B-20) Part III-B B-Series tables (B-2, B-3, B-6 to B-9, B-12 to B·24) Part IV Social and Cultural Tables Part IV-A C-Series tables (Tables C-'l to C--6, C-8) Part IV -B C.-Series tables (Table C-7, C-9, C-lO) Part V Migration Tables Part V-A D-Series tables (Tables D-l to D-ll, D-13, D-15 to D- 17) Part V-B D- Series tables (D - 12, D - 14) Part VI Fertility Tables F-Series tables (F-l to F-18) Part VII Tables on Houses and Household Amenities H-Series tables (H-I to H-6) Part VIII Special Tables on Scheduled Castes and Scheduled SC and ST series tables Tribes (SC-I to SC -14, ST -I to ST - 17) Part IX Town Directory, Survey report on towns and Vil Part IX-A Town Directory lages Part IX-B Survey Report on selected towns Part IX-C Survey Report on selected villages Part X Ethnographic notes and special studies on Sched uled Castes and Scheduled Tribes Part XI Census Atlas Publications of the Government of Goa Part XII District Census Handbook- one volume for each Part XII-A Village and Town Directory district Part XII-B Village and Town-wise Primary Census Abstract GOA A ADMINISTRATIVE DIVISIONS' 1991 ~. -

North Goa District Factbook |
Goa District Factbook™ North Goa District (Key Socio-economic Data of North Goa District, Goa) January, 2018 Editor & Director Dr. R.K. Thukral Research Editor Dr. Shafeeq Rahman Compiled, Researched and Published by Datanet India Pvt. Ltd. D-100, 1st Floor, Okhla Industrial Area, Phase-I, New Delhi-110020. Ph.: 91-11-43580781, 26810964-65-66 Email : [email protected] Website : www.districtsofindia.com Online Book Store : www.datanetindia-ebooks.com Also available at : Report No.: DFB/GA-585-0118 ISBN : 978-93-86683-80-9 First Edition : January, 2017 Second Edition : January, 2018 Price : Rs. 7500/- US$ 200 © 2018 Datanet India Pvt. Ltd. All rights reserved. No part of this book may be reproduced, stored in a retrieval system or transmitted in any form or by any means, mechanical photocopying, photographing, scanning, recording or otherwise without the prior written permission of the publisher. Please refer to Disclaimer & Terms of Use at page no. 208 for the use of this publication. Printed in India North Goa District at a Glance District came into Existence 30th May, 1987 District Headquarter Panaji Distance from State Capital NA Geographical Area (In Square km.) 1,736 (Ranks 1st in State and 522nd in India) Wastelands Area (In Square km.) 266 (2008-2009) Total Number of Households 1,79,085 Population 8,18,008 (Persons), 4,16,677 (Males), 4,01,331 (Females) (Ranks 1st in State and 480th in India) Population Growth Rate (2001- 7.84 (Persons), 7.25 (Males), 8.45 (Females) 2011) Number of Sub Sub-districts (06), Towns (47) and Villages (194) Districts/Towns/Villages Forest Cover (2015) 53.23% of Total Geographical Area Percentage of Urban/Rural 60.28 (Urban), 39.72 (Rural) Population Administrative Language Konkani Principal Languages (2001) Konkani (50.94%), Marathi (31.93%), Hindi (4.57%), Kannada (4.37%), Urdu (3.44%), Malayalam (1.00%) and Others (0.17%) Population Density 471 (Persons per Sq. -

Ecological Status and Management of Dr. Salim Ali Bird Sanctuary and Estuarine Areas of Chorao Island: a Desk Review CMPA Technical Report Series No
1 Ecological Status and Management of Dr. Salim Ali Bird Sanctuary and Estuarine Areas of Chorao Island: A Desk Review CMPA Technical Report Series No. 03 Ecological Status and Management of Dr. Salim Ali Bird Sanctuary and Estuarine Areas of Chorao Island: A Desk Review Authors Dr. Fraddry D’Souza, Asha Giriyan, Kavita Patil Published by Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH Indo-German Biodiversity Programme (IGBP), GIZ-India, A-2/18, Safdarjung Enclave, New Delhi - 110029, India E-mail: [email protected] Web: www.giz.de March 2015 Responsible Dr. Konrad Uebelhör, Director, GIZ Photo Credit Dr. Aaron Savio Lobo, Adviser, CMPA Design and layout Commons Collective, Bangalore [email protected] Disclaimer The views expressed in this document are solely those of the authors and may not in any circumstances be regarded as stating an official position of the Ministry of Environment, Forests and Climate Change (MoEFCC), Government of India, nor the German Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety (BMUB) or the Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH. The designation of geographical entities and presentation of material in this document do not imply the expression or opinion whatsoever on the part of MoEFCC, BMUB, or GIZ concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Reference herein to any specific organization, consulting firm, service provider or process followed does not necessarily constitute or imply its endorsement, recommendation or favouring by MoEFCC, BMUB or GIZ. -

CONTACT Email
NORTH GOA CONTACT Email IDs NO OF BEDS SOUTH GOA Email IDs CONTACT NO OF BEDS HOSPITALS Cottage Hospital [email protected] 2540864 Chicalim 60 Hospicio Hospital [email protected] 2705664/ North Goa District 2262291/ [email protected] 300 ,Margao 2705754 230 Hospital,Mapusa 2253387 Sub District Hospital [email protected] 2312115 220 ,Ponda T.B.Hospital [email protected] 2714866 68 COMMUNITY HEALTH CENTRES CHC Pernem 2201249 [email protected] 40 CHC Canacona [email protected] 2643422 70 CHC Valpoi 2375297 [email protected] 30 CHC Curchorem [email protected] 2650566 50 PRIMARY HEALTH CENTRES PHC Aldona 2293251 [email protected] 12 PHC Bali [email protected] 2670965 24 PHC Bicholim 2362041 [email protected] 30 PHC Cansaulim [email protected] 2754036 12 PHC candolim 2489035 [email protected] 12 PHC Chinchinim [email protected] 2863237 0 PHC Cansavornem 2205222 [email protected] 12 PHC Cortalim [email protected] 2550274 0 PHC colvale 2299870 [email protected] 0 PHC Curtorim [email protected] 2786206 12 PHC Corlim 2285769 [email protected] 0 PHC Loutalim [email protected] 2777140 0 PHC Sanquelim 2364258 [email protected] 70 PHC Quepum [email protected] 2662636 0 PHC Siolim 2272687 [email protected] 12 PHC Sanguem [email protected] 2604235 20 PHC Dharbandora [email protected] 2344221 12 PHC Ponda [email protected] 2319006 0 PHC Shiroda [email protected] 2307072 12 PHC Betki [email protected] 2287160 12 PHC Marcaim [email protected] 2392230 12 URBAN HEALTH CENTRES UHC Mapusa 2262226 [email protected] 0 UHC Margao [email protected] 2715004 0 UHC Panajim 2426495 [email protected] 0 UHC Vasco [email protected] 2512307 0 No. -

'Official ··'Gazette, ~
{R!;~D. OOA'-..~ l " Panaji, 26th Augusf, 1991 (Bhadra 4, 1913) . SERIES I No. 21 / 'OFFICIAL ··'GAZETTE, ~. " . - . - - . GOVERNMENT OF .····OOA EXTftl\OftD~ " / .' I NJ\ ftV ' GOVERNMENT OF GO~ chayats shall consist of such number of members as are indicated in the corresponding entry in column ;qeparlmenl of Community Developmenf and Panchayals . (5) of the said Schedule and further direct that the ntImller of'the ward in which a seat is reserved. for women shall be the one specified jn the correspond Notification ing entry in column (6) of the said Sc4edule. 1/15/(2)/90·CDP This Notification shallllave effect for the purpose · In exerci'se of the powers conferred by sub'section of the ensuing General Elections to all- the 183 Vil '.(1:) of section 3 and sub'sections.· (1) and (4) of . lage Panchayats as shown in the Said Schedule in ':section 7 of the Goa, Daman and Diu Village Pan cluding th'e 20 Village Panchayats whose terms have· cehayats Regulation, 1962 (No. 9 of 1962) (herein not expired but shall be co-terminus with .the Ge after called the 'said Regulation'), read with GO neral Election to Panchayats in terms of clause (b) vernment Notification No. 1/15(6)/84cF&A(3) of sub-section (6) of section 21 of the said Regula- · dated . 19c10-1987, I,. Shakti Sinha, Secretary, (Pim tion. .·chayats), Government of Goa, hereby declare the local areas comp.rising a village or group of villages. This Notification shall have effect for the purpose as shown in coluinn (4) of the Schedule appended of the' ensuing General Election to the VillagEr Pan hereto (hereinafter called the 'said ·,Schedule') to chayats and the existing constitution of Village shall · the' 'Viilage' as shown in the corresponding· entry continue till the date of the said elections on which in cohimn (3) of the said Schedule for. -
![OFFICIAL GOVERNMENT of GOA, DAMAN and DI\] Extrftordi N'f\RY](https://docslib.b-cdn.net/cover/5576/official-government-of-goa-daman-and-di-extrftordi-nf-ry-3775576.webp)
OFFICIAL GOVERNMENT of GOA, DAMAN and DI\] Extrftordi N'f\RY
I , BEGD. GOA· II I Panaji, 27th January, 1986 (Magha 7, 19071 SERies I·' No. 43 OFFICIAL GOVERNMENT OF GOA, DAMAN AND DI\] EXTRftORDI N'f\RY GOVERNM6NT . OF GOA, DAMAN column (3) of the said Schedule for the purpose.of establishment "f Village Panchayat under the said AND DIU Regulation and direct that the Village Panchayat shan have such number of members as are indicated Forest and Agriculture Department in the corresponding entry in column (5)cif'the said Schedule and further direct that the number of the ward' in which a seat for women is reserved shall be Notification the one .indicated in the corresponding entry in 1/15(19)/85-FA column (6) of the said Schedule. In exercise of the powers conferred by section 3 (i), Tllis Notification shall have effect for the purpose .7 and 9 of the Goa, Daman and Diu Village Pan· of the ensuing General Election to the Village Pan· chayats Regulation, 1962 (9 of 1962), read with the chayats an.d the existing constitution of Village shall . Government Notification No.. 1/15 (6)84-LAWD continue till the date of the said elections on which dated 9-1-1986, I, Denghnuna; Secretary (Co.,opera. date all earlier Notifications under which the Village tion and Social Welfare), Government of Goa, Daman Panchayats had been constituted shall cease to have and Diu, hereby declare that the local areas compri· effect. sed in the village or the group of villages shown in the colUinn (4) of .the Schedule appended hereto to be Denghnuna, Secretary (Co-Op. -

Seasonal Variations in Secondary Production Ofthe Mandovi-Zuari
Indian Journal of Marine Sciences Vol. 9, March 1980, pp. 7-9 Seasonal Variations in Secondary Production of the Mandovi-Zuari Estuarine System of Goa R A SELVAKUMAR, VIJAYALAKSHMI R NAIR & M MADHlJPRATAP National Institute of Oceanography, Dona Paula, Goa 403 ()()4 Received 16 April 1979: revised received 25 JUlie 1979 Mean rates of secondary production in the Mandovi, Zuari and Cumharjua canal were 16.9. 35.Q and 32.4 mg C/m2/day respectively. The general hydrographic conditions of the Zuari were responsible for the higher secondary production. In general, the saline period was more productive (24.9 mg dry wt/m ' /day) compared to the low saline period (II mg dry wt/m3/ day). The average secondary production in the estuarine system was 21.4 mg dry wl/m3jday or 1078 tonnes carbon/yr. The coefficient of energy transfer from primary to secondary level was 6.6%. Theoretical estimate of fish biomass in this estuarine system was 1007 tonnes/yr. The Mandovi and Zuari rivers and the interconnecting size 0.3 mm) with a flow meter attached. Surface water Cumbarjua canal form the major estuarine system of samples were collected for determining salinity. Dry Goa. Earlier reports indicate that the primary! and weight of samples was determined after removing benthic? productions in the estuary are quite high. larger organisms. A factor of 33.1~{, of dry weight 3 Studies on zooplankton from this area - 7 are limited estimated as the organic carbon content of estuarine to the abundance and diversity of different groups. In zooplankton" was used to compute the secondary this communication, an estimate of secondary production in terms of carbon. -

Cumbarjua Assembly Goa Factbook
Editor & Director Dr. R.K. Thukral Research Editor Dr. Shafeeq Rahman Compiled, Researched and Published by Datanet India Pvt. Ltd. D-100, 1st Floor, Okhla Industrial Area, Phase-I, New Delhi- 110020. Ph.: 91-11- 43580781-84 Email : [email protected] Website : www.indiastatelections.com Online Book Store : www.indiastatpublications.com Report No. : AFB/GA-15-0121 ISBN : 978-93-5313-471-6 First Edition : January, 2018 Third Updated Edition : January, 2021 Price : Rs. 11500/- US$ 310 © Datanet India Pvt. Ltd. All rights reserved. No part of this book may be reproduced, stored in a retrieval system or transmitted in any form or by any means, mechanical photocopying, photographing, scanning, recording or otherwise without the prior written permission of the publisher. Please refer to Disclaimer at page no. 75 for the use of this publication. Printed in India Contents No. Particulars Page No. Introduction 1 Assembly Constituency - (Vidhan Sabha) at a Glance | Features of Assembly 1-2 as per Delimitation Commission of India (2008) Location and Political Maps Location Map | Boundaries of Assembly Constituency - (Vidhan Sabha) in 2 District | Boundaries of Assembly Constituency under Parliamentary 3-10 Constituency - (Lok Sabha) | Town & Village-wise Winner Parties- 2019, 2017, 2012 and 2009 Administrative Setup 3 District | Sub-district | Towns | Villages | Inhabited Villages | Uninhabited 11-12 Villages | Village Panchayat | Intermediate Panchayat Demographics 4 Population | Households | Rural/Urban Population | Towns and Villages -
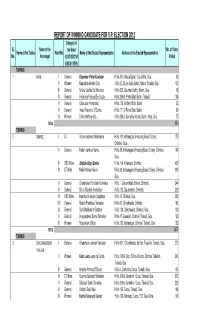
Corrected RESULT DATA
REPORT OF WINNING CANDIDATE FOR V.P. ELECTION 2012 Category of Sl. Name of the the Ward No. of Votes Name of the Taluka Ward No Name of the Elected Representative Address of the Elected Representative No. Panchayat (G/ST/OBC/W/ Polled OBCW/ STW) TISWADI-I 1 BATIM I General Digamber Vithal Kawlekar H.No.35/1, Maula Batim, Goa Velha, Goa 92 II Women Manuelina Alberto Dias H.No.22, Sluice Gate, Batim, Maina, Tiswadi, Goa. 135 III General Sneha Casilda De Menezes H.No.305, Gauncar Vaddo, Batim, Goa. 48 IV General Francisco Pascoal De Souza H.No.266/A, Portel Bhat, Batim, Tiswadi. 104 V General Celia Jose Fernandes H.No.130, Vodlem Bhatt, Batim. 52 VI General Irineo Francisco D'Cunha H.No.171/1, Ruma Bhat, Batim. 54 VII Women Emilia Anthony Vas H.No.263/1, Sovostkai Vaddo, Batim, Ilhas, Goa. 75 TOTAL 560 TISWADI-I 2 CHIMBEL I S.T. Rofam Kashinath Madkaikar H.No.101, Indiranagar & Housing Board Colony, 172 Chimbel, Goa. II General Mohan Laxman Narve H.No.38, Indiranagar & Housing Board Colony, Chimbel, 149 Goa. III OBC Wom. Shoban Arjun Borkar H.No.119, Kirlawada, Chimbel. 405 IV ST Wom. Mohini Mohan Narve H.No.38, Indiranagar & Housing Board Colony, Chimbel, 396 Goa. V General Chandrakant Pundalik Kunkolkar H.No.1, Gaulembhat, Shirent, Chimbel. 244 VI General Shruti Shailesh Kunkalikar H.No.132, Gaulembhat, Chimbel. 208 VII OBC Wom. Manisha Shailesh Chopdekar H.No.19, Chimbel, Goa. 298 VIII General Rajesh Prabhakar Vernekar H.No.61, Chinchwada, Chimbel. 181 IX General Sunil Madhukant Golatkar H.No.104, Chinchwada, Chimbel, Goa. -

OFFICIAL GAZETTE GOVERNMENT of GOA Extftf\ 0
{ REGD. GOA - 5 , Panaji, 12th October, 1992 (Asvina 20, 1914 SERIES II No. 28 OFFICIAL----------------------------- GAZETTE GOVERNMENT OF GOA EXTftf\ 0 ~D I NI\ ftT GOVERNMENT OF. GOA Category Clause Name of the members Educa'tion Department (v) Six Headmasters of Secendary scheols Goa Board of Secondary and Higher: Secondary frem six designated areas:- Education (1) Bardez: Shri Lambert Themas Car dezO', Notification St. Xavier's High School, No. GBSHSE/Brd-Ele/92-96/3327 Moira. In exercise of the pewers conferred by sub-section (2) ef (2) Pel'nem-Satari-Bicholim: section 12 of the Goa, Daman and Diu Secondary and Higher Shr.i Venkatesh Nilkant Secondary EducatiO'n Beard Act, 1975 (Act NO'. 13 of 1975), Natekar, read with Rule 29 of the Goa, Daman and Diu SecO'Didary and Higher Secendary Education BO'ard Members Election Proce Shri Sateri .English High dure Rules, 1979, the Goa, Daman and Diu BO'ard! of SecO'ndary Scheel, Ibrampur, Pernem. and Higher Secondary EducatiO'n hereby publishes the names ef persons, net being ex-officiO' membeTs, whO' have been (3) Ponda-8anguem: elected as members O'f the Board fO'r the term 1992-96. Shri Vasudeo Ramchandra Dhavalikar, Oa.tegO'ry Clause Name O'f the members Swastik Vidyalaya, PriO'l, Mardel. Class B: (ii) Four members to' represent the elected University O'f Goa:- (4) Quel!em-Canacona: Members Shri Sakharam Shivaram , (1) Sllri G. G. Bakhale, VeIguenker, St. Xavier's College, Mapusa. (2) Dr. K. P. Kamat, S. E. S. High Scheol, Cur Dean, Goa Dental CO'llege, chorem. -

CMPA-Technical-Report-Series-No
CMPA Technical Report Series No. 05 An Analysis of the Stakeholders in Dr. Salim Ali Bird Sanctuary, Chorao, Goa Author Solano Da Silva, Rayson K. Alex Namrata Namdeo Naik, Sushobhan Parida Published by Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH Indo-German Biodiversity Programme (IGBP), GIZ-India, A-2/18, Safdarjung Enclave, New Delhi - 110029, India E-Mail: [email protected] Web: www.giz.de November 2014 Responsible Dr. Konrad Uebelhör, Director, GIZ Photo Credit Dr. Aaron Savio Lobo, Adviser, GIZ Design and Layout Commons Collective, Bangalore [email protected] Disclaimer The views expressed in this document are solely those of the authors and may not in any circumstances be regarded as stating an official position of the Ministry of Environment, Forests and Climate Change (MoEFCC), Government of India, or of the German Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety (BMUB) or the Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH. The designation of geographical entities and presentation of material in this document do not imply the expression or opinion whatsoever on the part of MoEFCC, BMUB or GIZ concerning the legal or development status of any country, territory, city or area or of its authorities or concerning the delimitation of its frontiers or boundaries. Reference herein to any specific organisation, consulting firm, service provider or process followed does not necessarily constitute or imply its endorsement, recommendation or favouring by MoEFCC, BMUB or GIZ. Citation Da Silva, S., R K Alex, N N Naik, and S Parida. 2014. An Analysis of the Stakeholders in Dr Salim Ali Bird Sanctuary, Chorao, Goa.