A Multicenter Phase Ib/II Study of DNA-PK Inhibitor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Q2 2010 Half-Year Financial Report Worldreginfo - C49e8129-Cd51-43E5-Af66-E15ec3c851f8 2
Q2 2010 Half-Year Financial Report WorldReginfo - c49e8129-cd51-43e5-af66-e15ec3c851f8 2 CONTENTS 3 Half-Year Management Report of the Merck Group as of June 30, 2010 3 At a glance | Highlights 4 Merck Group 8 Merck shares 9 Business sectors 11 Divisions 11 Merck Serono 13 Consumer Health Care 15 Liquid Crystals 16 Performance & Life Science Chemicals 17 Merck Millipore 18 Corporate and Other 18 Risk Report 19 Report on Expected Developments 20 Half-Year Consolidated Financial Statements as of June 30, 2010 31 Responsibility Statement 32 Executive Board | Supervisory Board | Capital structure 33 Financial calendar for 2010 | Publication contributors Cover photo: Automated distribution of miniscule particles onto microtiter plates. These are used to synthesize compound libraries for ultra-high-throughput screening in drug discovery research. WorldReginfo - c49e8129-cd51-43e5-af66-e15ec3c851f8 Half-Year Management Report of the Merck Group as of June 30, 2010 3 At a glance | Highlights AT A glaNCE Key figures – 2nd quarter 2010 Pharma Corporate Change EUR million ceuticals Chemicals and Other Total in % Total revenues 1,564.4 643.6 – 2,208.0 15.6 Gross margin 1,297.2 393.5 – 1,690.8 21.5 Research and development –302.2 –36.5 –0.1 –338.8 –0.5 Operating result 158.2 166.0 2.0 326.2 76.8 Exceptional items – – –1.2 –1.2 – Earnings before interest and tax (EBIT) 158.2 166.0 0.8 325.0 76.2 EBIT before depreciation and amortization (EBITDA) 350.0 208.6 1.3 559.9 38.4 Return on sales in % (ROS: operating result/total revenues) 10.1 25.8 – 14.8 -

Unity: Concern Over Patent Licensing Draft .•.....• 1 Proposal on Cancelling Door-To-Door Sales Deals •....•
• Issue No. 421 Report No. 311, February 11, 1977 IN THIS ISSUE page Connnunity: Concern over Patent Licensing Draft .•.....• 1 Proposal on Cancelling Door-to-Door Sales Deals •....•. 2 Briefs: Court of Auditors; Fishing Licenses .•...•....• 3 Italy: 'Social Pact' Skirts Wage Cost Issue •........•. 3 Ireland: Budget Raises Speculation on Elections ....... 4 Britain: Modest Upturn Inspires Hopes, Caution ........ S France: Employers Want Shift in Social Cost Burden ..•. 6 Germany: Labor Costs Now Higher Than in USA .•.•••••••• 7 Euro Company Scene •••......•.....•.••..•.•..•....•. , •. 7 &unity: The European Connnission is going to great lengths to alle Business Fears viate the concern of the European business connnunity, es over Patent pecially in Germany and the U.K., over a preliminary draft Licensing Draft regulation that would exempt en bloc from the Article 85(1) ban any licensing agreements containing certain restrictive clauses. The Connnission and national experts were sched uled to discuss the document again this month, before in viting connnents from national business associations. In the meantime, however, Brussels has been taken by surprise by the publication of the confidential preliminary draft in a respected German financial newspaper. Connnission offi cials are now trying to counter criticism of business rep resentatives, who have described the draft as being "too narrowly conceived, contradictory, and impractical." The regulation would relieve the parties both from no tifying the Connnission and from applying for an exemption for a restrictive licensing agreement (as they are required to do now) if the latter meets the criteria set out in the draft regulation. A block exemption would greatly ease the Connnission's work load because the applications for exemp tions have been piling up by the tens of thousands. -

The Resiliency Compass: Navigating Global Value Chain Disruption in an Age of Uncertainty
In Collaboration with Kearney The Resiliency Compass: Navigating Global Value Chain Disruption in an Age of Uncertainty WHITE PAPER JULY 2021 Images: Getty Images, Unsplash Contents Foreword 3 Executive summary 4 1 Disruption drives a rethink 5 2 Resilience in action 8 2.1 Getting to grips with disruption 8 2.2 Introducing the resiliency compass 8 2.3 Who are the resilience leaders? 10 3 Setting the right course with the resiliency compass 11 4 Call for action: global coordination for the long term 14 Methodology 15 Contributors 16 Acknowledgements 17 Endnotes 19 © 2021 World Economic Forum. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, including photocopying and recording, or by any information storage and retrieval system. The Resiliency Compass: Navigating Global Value Chain Disruption in an Age of Uncertainty 2 July 2021 The Resiliency Compass: Navigating Global Value Chain Disruption in an Age of Uncertainty Foreword Unlocking the future of cooperation, resilience and prosperity for global value chains. Francisco Betti Per Kristian Hong Head of Shaping the Future of Managing Director and Advanced Manufacturing and Partner, Strategic Operations Production, Member of the and World Economic Forum Executive Committee, World Relationship Lead, Kearney Economic Forum COVID-19 has kept manufacturing companies systems if both the global economy and companies beyond busy for many months and the challenges are to successfully navigate future disruptions that are far from over, from ensuring safety and may affect global value chains. security on the shop floor and facing supply and demand disruptions to accelerating digital In 2020, the World Economic Forum, in transformation and reskilling to build resilience. -

Disclaimerthis Is a Publication of a Third Party
DisclaimerThis is a publication of a third party. Merck KGaA, Darmstadt, Germany is not responsible for its content. Whenever this document refers to Merck it refers to Merck KGaA, Darmstadt, Germany or the group of companies affiliated with Merck KGaA, Darmstadt, Germany. In the United States and Canada the group of companies affiliated with Merck KGaA, Darmstadt, Germany operates under individual business names (EMD Serono, MilliporeSigma, EMD Performance Materials). 2 November 2018 Corporates Merck KGaA Merck KGaAGermany, Chemicals/Pharmaceuticals STABLE Germany, Chemicals/Pharmaceuticals A- Corporate profile Ratings & Outlook Merck KGaA is a diversified chemicals/pharmaceuticals group that was founded in 1668 Corporate Ratings A-/Stable with the opening of a Merck pharmacy in Darmstadt, Germany, where the group is still Short Term Rating S-1/Stable based. The Merck family holds 70% of the voting rights with the remainder in public ownership. After several acquisitions and divestments, the group now consists of three divisions: healthcare (pharmaceuticals and consumer healthcare), life science and Analysts performance materials (centred on its global market leadership in liquid crystals). In 2015, Olaf Tölke Merck acquired the US-based life science company Sigma Aldrich for a price of +49 69 6677389 11 USD 17bn and became one of the consolidators in that industry. In pharmaceuticals, it is [email protected] a specialised, mid-sized producer of drugs, relying on two blockbuster products, Erbitux and Rebif, but has developed a new focus on immuno-oncological products, mainly Related methodology represented by its antibody avelumab. At the end of 2014, US-based big pharma Corporate Rating Methodology, company Pfizer acquired partial ownership of the molecule as well as US distribution January 2018 rights for USD 850m. -

Dr. Holger Hofmeister
Dr. Holger Hofmeister Partner, Frankfurt Mergers and Acquisitions Dr. Holger Hofmeister advises corporate clients and private equity firms in connection with acquisitions, divestitures, public takeovers, joint ventures, and other M&A and corporate matters, including capital markets-related issues. In addition, he advises on corporate governance-related matters and corporate restructurings. Dr. Hofmeister was named in WirtschaftsWoche as one of the best corporate lawyers in Germany. In addition, he has been named by both Handelsblatt and WirtschaftsWoche as one of Germany’s top M&A advisors. He also is listed as recommended lawyer in JUVE and was listed in Best Lawyers in Germany 2021. Dr. Hofmeister’s representative matters include advising, among others: - Adevinta ASA in its US$9.2 billion acquisition of eBay Classifieds Group from eBay Inc.; T: 49.69.74220.117 - Airbnb, Inc. in its acquisition of Germany-based fewoVista S.L. & Co. KG and Vacaleo GmbH; F: 49.69.74220.417 - Apax Partners in connection with: [email protected] • its proposed US$8.5 billion bid for ISS/AS, a facilities management provider, from Goldman Sachs Capital Partners and EQT Partners AB; and Education • the US$420 million sale of Versatel AG, a provider of telecommunications and informa- Second State Exam, Appellate Court tion technology services, to Kohlberg Kravis Roberts & Co., a private equity firm; of Duesseldorf, 2001 - Black Diamond Capital Management, L.L.C., along with InvestIndustrial, in their Dr. jur., University of Regensburg, 1999 US$425 million acquisition of the phenolic specialty resin, hexamine and European forest products resins businesses of Hexion Inc.; First State Exam, University of Regensburg, 1998 - Braskem S.A. -
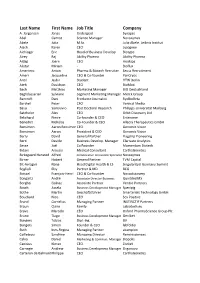
Last Name First Name Job Title Company A
Last Name First Name Job Title Company A. Jürgensen Jonas Undergrad Synapse Abel Gernot Science Manager Novozymes Abele Julia M.Sc. Julia Abele, Leibniz Institut Aiach Karen CEO Lysogene Aichinger Eric Head of Business Develop. Biotype Airey Raj Ability Pharma Ability Pharma Aldag Joern CEO Hookipa Alistar Miriam - Bioflux Ameniero Anton Pharma & Biotech Recruiter Seuss Recruitment Ameri JacQueline CEO & Co-founder PanCryos Antil Jasbir Student HTW Berlin Ateh Davidson CEO BioMoti Bach Matthias Marketing Manager BIO Deutschland Baghdassarian Sylviane Segment Marketing Manager Merck Group Bancroft Dani Freelance Journalist SynBioBeta Barthel Peter CFO Vertical Media Basu Somsuvro Post Doctoral Research Philipps-Universität Marburg Batchelor Alex CEO Orbit Discovery Ltd Belichard Pierre Co-founder & CEO Enterome Benedict Nicholas Co-Founder & CEO Allecra Therapeutics GmbH Bensimon Aaron BensimonCEO Genomic Vision Bensimon Aaron President & CEO Genomic Vision Berry David General Partner Flagship Pioneering Berti Davide Business Develop. Manager Clarivate Analytics Besse Joël Co Founder Momentum Biotech Bidani Aroussi Medical Consultant CarthaGenetics Birkegaard Hansted Alfred Collaborative Innovation Specialist Novozymes Birner Hubert General Partner TVM Capital Bit-Avragim Nana Head Digital Health & LS SingularityU Germany Summit Boglioli Elsy Partner & MD BCG Boissel François-Henri CEO & Co-founder Novadiscovery Bongartz André Associate Director Business QuintilesIMS Borghei Golnaz Associate Partner Ventac Partners Bosch Assela Business Development Manager Specipig Bothe Martin Geschäftsführer Smarterials Technology GmbH Bouchard Nico CEO Sex Positive Brand Cornelius Managing Partner INSTINCTIF Partners Braun Claire Family Labiotech.eu Bravo Marcelo CEO Oxford PharmaScience Group Plc Bruno Tiago Business Development Manager GenIbet Bungers Tobias Dipl.-Ing. BV Bungers Simon Founder & CEO labfolder Buth Ines-Regina Managing Partner akampion Cailleteau Sandrine Managing Director Reminisciences Calvo Daniel Marketing & Commun. -

2.2.2. List of Corporate Offices and Directorships of the Directors Exercised at 31 December 2020
CORPORATE GOVERNANCE Composition of the Board of Directors 2.2.2. List of corporate offices and directorships of the Directors exercised at 31 December 2020 JEAN-PAUL AGON Chairman and Chief Executive Officer French Age: 64 Joined the L’Oréal Group in 1978. Following an international career as Chief Executive Officer of the Consumer Products 2 Division in Greece and of L’Oréal Paris in France, International Managing Director of Biotherm, Chief Executive Officer of L’Oréal Germany, Chief Executive Officer of the Asia Zone, Chairman and Chief Executive Officer of L’Oréal USA, Jean-Paul Agon was appointed as Deputy Chief Executive Officer of L’Oréal in 2005, and then Chief Executive Officer in April 2006 and finally Chairman and Chief Executive Officer in 2011. A Director of L’Oréal since 2006, he is also the Chairman of the Strategy and Sustainability Committee. Jean-Paul Agon is also the Chairman of the L’Oréal Corporate Foundation and Director of Air Liquide. By decision of the Board of Directors following the 2021 Annual General Meeting, Jean-Paul Agon will hold the office of Chairman of the Board of Directors without assuming the office of Chief Executive Officer as from 1 May 2021. EXPIRY DATE OF TERM OF OFFICE: 2022 ● Professional address: L’Oréal – 41, rue Martre – 92117 Clichy Cedex – France ● Holds 1,365,432 L’Oréal shares OTHER CORPORATE OFFICES AND DIRECTORSHIPS HELD French companies Air Liquide S.A.* Director Other L’Oréal Corporate Foundation Chairman of the Board L’Oréal Fund for Women Chairman of the Board Raisesherpas Director French Association of Private Enterprises (AFEP) Director French Institute of International Relations (IFRI) Director CORPORATE OFFICES AND DIRECTORSHIPS HELD OVER THE LAST FIVE YEARS AND EXPIRED None * Listed company. -

GC Report 2011-En.Pdf
BASF’s segments BASF in Greater China Report 2011 Chemicals Functional Solutions Content In the Chemicals segment, we supply Key data Chemicals (million €) In the Functional Solutions segment, we Key data Functional Solutions (million €) products to customers in the chemical, bundle system solutions and innovative About the report 01 Environment and safety 26 electronics, construction, textile, auto- 2011 2010 Change in % products for specific sectors and custo- 2011 2010 Change in % motive, pharmaceutical and agricultural Sales 12,958 11,377 13.9 mers, in particular for the automotive, Sales 11,361 9,703 17.1 BASF Group 02 Society 33 industries as well as many others. We chemical and construction industries. also ensure that other BASF segments EBITDA 3,188 3,000 6.3 Our portfolio comprises automotive and EBITDA 921 861 7.0 BASF in Asia Pacific 06 Recognition 38 are supplied with chemicals for produ- Income from operations industrial catalysts, automotive and Income from operations 2,441 2,302 6.0 559 467 19.7 cing downstream products. Our portfolio before special items industrial coatings and concrete admix- before special items Driving sustainability in Greater China 08 BASF in the regions 40 ranges from basic chemicals, glues and Income from operations tures as well as construction systems Income from operations 2,442 2,310 5.7 427 457 (6.6) electronic chemicals for the semicon- (EBIT) such as tile adhesives and architectural (EBIT) BASF in Greater China 10 Ten-year summary 42 ductor and solar cell industries, to coatings. solvents and plasticizers, as well as Strategic areas 14 Business contacts in Greater China 43 starting materials for detergents, 1 3 3 plastics, textile fibers, paints and Sales (%) Sales (%) Innovation 22 BASF’s segments at a glance inside front cover coatings, and pharmaceuticals. -

Chief Technology Officer of BASF Group to Keynote the CIO Germany, Austria and Switzerland Summit
Press Contacts: Rachel Tait Director, Client Services & Event Management CDM Media [email protected] +1 312.374.0800 Kieran Hasan Director, EMEA, CIO Summits CDM Media [email protected] +44.2920.729.275 Chief Technology Officer of BASF Group to Keynote the CIO Germany, Austria and Switzerland Summit CHICAGO, April 24, 2014 – CDM Media announced Wiebe Van Der Horst, Senior Vice President, Global Process & Enterprise Architecture, and Chief Technology Officer of the BASF Group, will be the opening keynote speaker at the second annual CIO Germany, Austria and Switzerland Summit, which will be held September 17, 2014 in Frankfurt, Germany. The CIO Germany, Austria and Switzerland Summit offers a unique platform for the DACH region’s foremost technology thinkers to collaborate on the latest industry topics and trends. The agenda’s format is designed for attending executives to discuss and share insight on the issues currently impacting CIOs in the region, while learning about the leading-edge products and services. Mr. Van Der Horst brings 20 years of experience and knowledge working in supply chain management, procurement and information systems. He will be kicking of the summit with an opening keynote presentation entitled, “From the hunted to the hunter – Transforming Information Services (IS) from reactive cost center to the proactive business enabler.” Mr. Van Der Horst will discuss how BASF has leveraged 4 key enablers (enterprise architecture, focus on end-to-end processes, change management and emerging technologies) to become a business solutions provider. “Having the BASF Group represented at the summit is outstanding, and having Wiebe Van Der Horst keynote the summit will promise for a very valuable and content-rich event,” said Kieran Hasan, Director EMEA, CIO Summits. -

MERCK Kgaa, DARMSTADT, GERMANY Annual Financial Statements 2017 DISCLAIMER
350 MERCK KGaA, DARMSTADT, GERMANY Annual Financial Statements 2017 DISCLAIMER Publication of Merck KGaA, Darmstadt, Germany. In the United States and Canada the subsidiaries of Merck KGaA, Darmstadt, Germany, operate as EMD Serono in Healthcare, MilliporeSigma in Life Science and EMD Performance Materials. To reflect such fact and to avoid any misconception of the reader of the publication certain logos, terms and names of businesses of the publication have been substituted or additional descriptions have been added. This version of the publication, therefore, slightly deviates from the otherwise identical version of the publication provided outside the United States and Canada. ALWAYS CURIOUS I AGINE THE NEXT 350 YEARS We have been demonstrating our innovative strength for 350 years. Curiosity connects us. It drives human progress – and challenges us to imagine the next 350 years. Table of contents Combined Management Report 004 – 114 Annual Financial Statements 005 Fundamental Information 116 – 170 about the Group 116 Balance Sheet as of 005 The Group December 31, 2017 012 Objectives and strategies 117 Income Statement from the period 018 Internal Management System from January 1 to December 31, 2017 022 Corporate Responsibility 119 Notes to the Annual Financial Statements 031 Research and development 119 General Disclosures 042 People 121 Notes to the Balance Sheet 130 Notes to the Income Statement 048 Report on Economic Position 134 Other Disclosures 048 Macroeconomic and sector-specific environment 138 Members of the Executive Board of Merck KGaA, Darmstadt, Germany 051 Review of Forecast against Actual Business Developments 139 Supervisory Board 058 Course of business and 140 Disclosures in accordance with section economic position 160 (1) no. -

Short CV Dr. Wolfgang Büchele Name: Qualification
Short CV Dr. Wolfgang Büchele Name: Dr. Wolfgang Büchele Qualification: Chemist Current occupation: Chief Executive Officer Exyte GmbH, Stuttgart, Germany Geburtsdatum: August 11, 1959 Nationality: German Education: University degree course in chemistry, University of Ulm (concentration in solid state chemistry), Degree: Graduate Chemist 1979-1984 Assistant in inorganic chemistry, University of Ulm under Professor Dr. rer. nat. Lentz Doctorate (Dr. rer. nat) with a specialization in 1984-1987 inorganic chemistry 1987 Research chemist for industrial catalysts Professional career: Research group head (industrial catalysts) BASF AG Head of Regional Marketing Catalysts Asia, Hong 1987-1990 Kong 1990-1993 Head of Global Marketing Cosmetic Raw Materials 1993-1997 Head of Business Management Fine Chemicals Europe 1997-1999 President of Eastern Europe, Africa, West Asia 1999-2001 Regional Division President, Performance Chemicals 2001-2003 Supervisory Board Chairman BASF Drucksysteme GmbH, Stuttgart, Germany 2003-2005 1 of 3 2003-2007 Member of the Site Management Committee of the Ludwigshafen site 2005-2007 President, Fine Chemicals 2007 Appointed to the Management Board effective January 1, 2008 with responsibility for oil and gas, the region Europe as well as Global Procurement & Logistics The Blackstone Group LLP 2008 Project Advisor (incl. minority interest in Evonik AG) Permira Beteiligungsberatung GmbH, Frankfurt, Germany 2008-2011 Senior Advisor BorsodChem Zrt. , Kazincbarcika/Ungarn 2008-2011 Chief Executive Officer 2009-2011 Chief Executive -

Future Workforce Planning Summit.Digital NEU: 5
DGFP // HUMAN Future Workforce Planning Summit.digital NEU: 5. November 2020, 10:00–16:15 Uhr als Online- Konferenz im November In Kooperation mit Unsere Highlights: // Kosten runter, Transformation rauf: So steuern Sie Ihre Personal struktur auf dem Weg ins „New Normal“. // Strategische Personalplanung als Kriseninstrument: So setzen Sie SPP schnell und effektiv um. // Future Skills: Hier erfahren Sie, welche Kompetenzen Ihr Unter nehmen in Zukunft braucht. // Wissen aus der Praxis: zwei Vorstands und Mittelstandspanels, vier Breakouts mit CHROs und Experten von Continental, Deutsche Bahn, Deutsche Telekom, HeidelbergCement, Infineon, KnorrBremse, Merck, Siemens, Trumpf u. v. a. www.dgfp.de Liebe DGFP // Community, die Coronakrise bringt für viele Unternehmen das Thema Steuerung der Personalkosten zurück auf die Agenda, um so das Unternehmen sicher aus der Krise zu navigieren. Der durch die wirtschaftliche Situation notwendig gewordene Umbau der Belegschaften wird durch den digitalen Wandel noch verstärkt. So braucht es in bestimmten Branchen und Unter nehmen weniger Beschäftigte, gleichzeitig aber völlig neue Kompetenzen. Mit dem Instrument der Strategischen Personalplanung (SPP) lässt sich diese Transformation weiter vorantreiben. Doch wie funktioniert SPP in der Praxis konkret? Welche Ansätze haben sich bewährt, vor allem auch bei kleineren Budgets? Wo liegt der Wertbeitrag und wo die Hürden? Wir geben Ihnen Antworten! Seien Sie dabei, wenn sich Experten und HRCommunity am 5. November 2020 digital zum 3. Future Workforce Planning Summit der Deutschen Gesellschaft für Personalführung e.V. und der Unternehmensberatung HUMAN treffen. Referenten von Continental, Deutsche Bahn, Deutsche Telekom, HeidelbergCement, Infineon, KnorrBremse, Merck, Siemens, Trumpf. u. v a. liefern die passenden Impulse aus der Unternehmenspraxis und zeigen Trends und Entwicklungen der StateoftheArtPersonalplanung und des WorkforceManagements.