Confidential: for Review Only Hydroxychloroquine in Patients Mainly with Mild to Moderate COVID–19: an Open–Label, Randomized, Controlled Trial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Landscape Analysis of Geographical Names in Hubei Province, China
Entropy 2014, 16, 6313-6337; doi:10.3390/e16126313 OPEN ACCESS entropy ISSN 1099-4300 www.mdpi.com/journal/entropy Article Landscape Analysis of Geographical Names in Hubei Province, China Xixi Chen 1, Tao Hu 1, Fu Ren 1,2,*, Deng Chen 1, Lan Li 1 and Nan Gao 1 1 School of Resource and Environment Science, Wuhan University, Luoyu Road 129, Wuhan 430079, China; E-Mails: [email protected] (X.C.); [email protected] (T.H.); [email protected] (D.C.); [email protected] (L.L.); [email protected] (N.G.) 2 Key Laboratory of Geographical Information System, Ministry of Education, Wuhan University, Luoyu Road 129, Wuhan 430079, China * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel: +86-27-87664557; Fax: +86-27-68778893. External Editor: Hwa-Lung Yu Received: 20 July 2014; in revised form: 31 October 2014 / Accepted: 26 November 2014 / Published: 1 December 2014 Abstract: Hubei Province is the hub of communications in central China, which directly determines its strategic position in the country’s development. Additionally, Hubei Province is well-known for its diverse landforms, including mountains, hills, mounds and plains. This area is called “The Province of Thousand Lakes” due to the abundance of water resources. Geographical names are exclusive names given to physical or anthropogenic geographic entities at specific spatial locations and are important signs by which humans understand natural and human activities. In this study, geographic information systems (GIS) technology is adopted to establish a geodatabase of geographical names with particular characteristics in Hubei Province and extract certain geomorphologic and environmental factors. -

Download Article
Advances in Economics, Business and Management Research, volume 70 International Conference on Economy, Management and Entrepreneurship(ICOEME 2018) Research on the Path of Deep Fusion and Integration Development of Wuhan and Ezhou Lijiang Zhao Chengxiu Teng School of Public Administration School of Public Administration Zhongnan University of Economics and Law Zhongnan University of Economics and Law Wuhan, China 430073 Wuhan, China 430073 Abstract—The integration development of Wuhan and urban integration of Wuhan and Hubei, rely on and Ezhou is a strategic task in Hubei Province. It is of great undertake Wuhan. Ezhou City takes the initiative to revise significance to enhance the primacy of provincial capital, form the overall urban and rural plan. Ezhou’s transportation a new pattern of productivity allocation, drive the development infrastructure is connected to the traffic artery of Wuhan in of provincial economy and upgrade the competitiveness of an all-around and three-dimensional way. At present, there provincial-level administrative regions. This paper discusses are 3 interconnected expressways including Shanghai- the path of deep integration development of Wuhan and Ezhou Chengdu expressway, Wuhan-Ezhou expressway and from the aspects of history, geography, politics and economy, Wugang expressway. In terms of market access, Wuhan East and puts forward some suggestions on relevant management Lake Development Zone and Ezhou Gedian Development principles and policies. Zone try out market access cooperation, and enterprises Keywords—urban regional cooperation; integration registered in Ezhou can be named with “Wuhan”. development; path III. THE SPACE FOR IMPROVEMENT IN THE INTEGRATION I. INTRODUCTION DEVELOPMENT OF WUHAN AND EZHOU Exploring the path of leapfrog development in inland The degree of integration development of Wuhan and areas is a common issue for the vast areas (that is to say, 500 Ezhou is lower than that of central urban area of Wuhan, and kilometers from the coastline) of China’s hinterland. -

A Simple Model to Assess Wuhan Lock-Down Effect and Region Efforts
A simple model to assess Wuhan lock-down effect and region efforts during COVID-19 epidemic in China Mainland Zheming Yuan#, Yi Xiao#, Zhijun Dai, Jianjun Huang & Yuan Chen* Hunan Engineering & Technology Research Centre for Agricultural Big Data Analysis & Decision-making, Hunan Agricultural University, Changsha, Hunan, 410128, China. #These authors contributed equally to this work. * Correspondence and requests for materials should be addressed to Y.C. (email: [email protected]) (Submitted: 29 February 2020 – Published online: 2 March 2020) DISCLAIMER This paper was submitted to the Bulletin of the World Health Organization and was posted to the COVID-19 open site, according to the protocol for public health emergencies for international concern as described in Vasee Moorthy et al. (http://dx.doi.org/10.2471/BLT.20.251561). The information herein is available for unrestricted use, distribution and reproduction in any medium, provided that the original work is properly cited as indicated by the Creative Commons Attribution 3.0 Intergovernmental Organizations licence (CC BY IGO 3.0). RECOMMENDED CITATION Yuan Z, Xiao Y, Dai Z, Huang J & Chen Y. A simple model to assess Wuhan lock-down effect and region efforts during COVID-19 epidemic in China Mainland [Preprint]. Bull World Health Organ. E-pub: 02 March 2020. doi: http://dx.doi.org/10.2471/BLT.20.254045 Abstract: Since COVID-19 emerged in early December, 2019 in Wuhan and swept across China Mainland, a series of large-scale public health interventions, especially Wuhan lock-down combined with nationwide traffic restrictions and Stay At Home Movement, have been taken by the government to control the epidemic. -

Encephalomyelitis Ameliorates Experimental Autoimmune And
The Journal of Immunology Vibsanin B Preferentially Targets HSP90b, Inhibits Interstitial Leukocyte Migration, and Ameliorates Experimental Autoimmune Encephalomyelitis Bai-Xin Ye,*,1 Xu Deng,†,1 Li-Dong Shao,† Ying Lu,* Run Xiao,* Yi-Jie Liu,* Yi Jin,* Yin-Yin Xie,* Yan Zhao,* Liu-Fei Luo,* Shun Ma,‡ Ming Gao,x Lian-Ru Zhang,‡ Juan He,† Wei-Na Zhang,* Yi Chen,* Cheng-Feng Xia,† Min Deng,{ Ting-Xi Liu,*,{ Qin-Shi Zhao,† Sai-Juan Chen,* and Zhu Chen* Interstitial leukocyte migration plays a critical role in inflammation and offers a therapeutic target for treating inflammation- associated diseases such as multiple sclerosis. Identifying small molecules to inhibit undesired leukocyte migration provides promise for the treatment of these disorders. In this study, we identified vibsanin B, a novel macrocyclic diterpenoid isolated from Viburnum odoratissimum Ker-Gawl, that inhibited zebrafish interstitial leukocyte migration using a transgenic zebrafish line (TG:zlyz– enhanced GFP). We found that vibsanin B preferentially binds to heat shock protein (HSP)90b. At the molecular level, inacti- vation of HSP90 can mimic vibsanin B’s effect of inhibiting interstitial leukocyte migration. Furthermore, we demonstrated that vibsanin B ameliorates experimental autoimmune encephalomyelitis in mice with pathological manifestation of decreased leuko- cyte infiltration into their CNS. In summary, vibsanin B is a novel lead compound that preferentially targets HSP90b and inhibits interstitial leukocyte migration, offering a promising drug lead for treating inflammation-associated diseases. The Journal of Immunology, 2015, 194: 4489–4497. eukocyte trafficking is a multistep process consisting of moattractant signals, plays an important role in immune cell de- transendothelial and interstitial migration of leukocytes, velopment, immunosurveillance, and effector functions. -

Study on Intangible Cultural Heritage Brands from the Perspective Of
International Conference on Education Technology, Management and Humanities Science (ETMHS 2015) Study on Intangible Cultural Heritage Brands from the Perspective of Ecological Protection Haimeng Li School of music, Hubei Normal University, Huangshi, 435002, China Keywords: ecological protection, intangible cultural heritage, brands Abstract: China has abundant intangible cultural heritage resources. However, economic development brings pressure to ecological environment, changes of which have direct influence on the protection of intangible cultural heritage. At current stage, due to lack of strong consciousness of intangible cultural heritage protection in many regions in China, a part of intangible cultural heritage is in imminent danger. Establishment of intangible cultural heritage brands means inheritance and development of intangible cultural heritage, and is also a way of intangible cultural heritage protection. This paper mainly studies on intangible cultural heritage brands from the perspective of ecological protection with an example of protection of intangible cultural heritage in Southeastern Hubei Province. As intangible cultural heritage has tourism value, various countries begin to attach importance to tourism development of intangible cultural heritage. However, unreasonable development is caused for many factors, which cannot inherit or develop intangible cultural heritage but lead to frequent problems in its protection. Establishment of intangible cultural heritage brands is an important way of intangible cultural development -

Are China's Water Resources for Agriculture Sustainable? Evidence from Hubei Province
sustainability Article Are China’s Water Resources for Agriculture Sustainable? Evidence from Hubei Province Hao Jin and Shuai Huang * School of Public Economics and Administration, Shanghai University of Finance and Economics, Shanghai 200433, China; [email protected] * Correspondence: [email protected]; Tel.: +86-21-65903686 Abstract: We assessed the sustainability of agricultural water resources in Hubei Province, a typical agricultural province in central China, for a decade (2008–2018). Since traditional evaluation models often consider only the distance between the evaluation point and the positive or negative ideal solution, we introduce gray correlation analysis and construct a new sustainability evaluation model. Our research results show that only one city had excellent sustainable development capacity of agricultural water resources, and the evaluation value of eight cities fluctuated by around 0.5 (the median of the evaluation result), while the sustainable development capacity of agricultural water resources in other cities was relatively poor. Our findings not only reflect the differences in the natural conditions of water resources among various cities in Hubei, but also the impact of the cities’ policies to ensure efficient agricultural water use for sustainable development. The indicators and methods Citation: Jin, H.; Huang, S. Are in this research are not difficult to obtain in most countries and regions of the world. Therefore, the China’s Water Resources for indicator system we have established by this research could be used to study the sustainability of Agriculture Sustainable? Evidence agricultural water resources in other countries, regions, or cities. from Hubei Province. Sustainability 2021, 13, 3510. https://doi.org/ Keywords: water resources; agricultural water resources; sustainability; gray correlation analysis; 10.3390/su13063510 evaluation model Academic Editors: Daniela Malcangio, Alan Cuthbertson, Juan 1. -
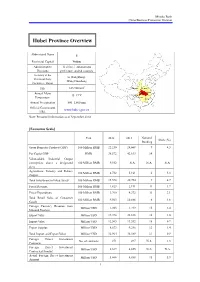
Hubei Province Overview
Mizuho Bank China Business Promotion Division Hubei Province Overview Abbreviated Name E Provincial Capital Wuhan Administrative 12 cities, 1 autonomous Divisions prefecture, and 64 counties Secretary of the Li Hongzhong; Provincial Party Wang Guosheng Committee; Mayor 2 Size 185,900 km Shaanxi Henan Annual Mean Hubei Anhui 15–17°C Chongqing Temperature Hunan Jiangxi Annual Precipitation 800–1,600 mm Official Government www.hubei.gov.cn URL Note: Personnel information as of September 2014 [Economic Scale] Unit 2012 2013 National Share (%) Ranking Gross Domestic Product (GDP) 100 Million RMB 22,250 24,668 9 4.3 Per Capita GDP RMB 38,572 42,613 14 - Value-added Industrial Output (enterprises above a designated 100 Million RMB 9,552 N.A. N.A. N.A. size) Agriculture, Forestry and Fishery 100 Million RMB 4,732 5,161 6 5.3 Output Total Investment in Fixed Assets 100 Million RMB 15,578 20,754 9 4.7 Fiscal Revenue 100 Million RMB 1,823 2,191 11 1.7 Fiscal Expenditure 100 Million RMB 3,760 4,372 11 3.1 Total Retail Sales of Consumer 100 Million RMB 9,563 10,886 6 4.6 Goods Foreign Currency Revenue from Million USD 1,203 1,219 15 2.4 Inbound Tourism Export Value Million USD 19,398 22,838 16 1.0 Import Value Million USD 12,565 13,552 18 0.7 Export Surplus Million USD 6,833 9,286 12 1.4 Total Import and Export Value Million USD 31,964 36,389 17 0.9 Foreign Direct Investment No. -

Acupuncture in China
Acupuncture in China A Suggested Curriculum for a 4th-Year Clinical Rotation April 14, 2011 Ben Mervak, Alison Kalinowski, and Malani Gupta Table of Contents A LETTER OF INTRODUCTION ................................................................................................ 2 SUGGESTED LEARNING OBJECTIVES .......................................................................................... 3 INTRODUCTION TO ACUPUNCTURE AND RELATED THERAPIES .......................................................... 4 PATIENT DEMOGRAPHICS ..................................................................................................... 7 SAMPLE AM CLINIC PATIENT LOG ........................................................................................ 8 SAMPLE AM CLINIC PROCEDURE LOG ................................................................................... 9 CASE REPORT .................................................................................................................. 10 1. PLACEMENT OF SCALP NEEDLES ....................................................................................... 11 2. PLACEMENT OF UPPER EXTREMITY NEEDLES ......................................................................... 11 3. PLACEMENT OF ABDOMINAL NEEDLES ................................................................................ 12 4. PLACEMENT OF LOWER EXTREMITY NEEDLES........................................................................ 13 RECOMMENDED REQUIREMENTS FOR FUTURE ROTATORS ............................................................. -
Orthotopic Liver Transplantation for Primary Intrahepatic Adenosquamous Carcinoma Using a Marginal Graft: the First Case Rrport
Orthotopic Liver Transplantation for Primary Intrahepatic Adenosquamous Carcinoma Using A Marginal Graft: the First Case rRport Peng Chen Shanghai Jiao Tong University Medical School Aliated Ruijin Hospital Di Ma Shanghai Jiao Tong University Medical School Aliated Ruijin Hospital Ruokun Li Shanghai Jiao Tong University Medical School Aliated Ruijin Hospital Xiaoqun Yang Shanghai Jiao Tong University Medical School Aliated Ruijin Hospital Hui Tong Shanghai Jiao Tong University Medical School Aliated Ruijin Hospital Debin Qi Shanghai Jiao Tong University Medical School Aliated Ruijin Hospital Tao Li ( [email protected] ) Shanghai Jiao Tong University Medical School Aliated Ruijin Hospital https://orcid.org/0000-0003- 4035-0729 Case report Keywords: case report, cancer/malignancy/neoplasia, primary adenosquamous carcinoma, cholangiocarcinoma, liver transplantation/hepatology Posted Date: December 2nd, 2020 DOI: https://doi.org/10.21203/rs.3.rs-117738/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/17 Abstract Background. Primary intrahepatic adenosquamous carcinoma (iASC) is a very rare subtype of cholangiocarcinoma (CCA), with a worse prognosis than adenocarcinoma. Hepatectomy is still the rst choice for iASC, and liver transplantation (LT) for iASC has not been previously reported. Case presentation The young male patient with an unresectable primary iASC underwent a LT using a marginal fatty liver graft. The overall survival of this patient was 16 months, with a recurrence-free survival of 8 months. The patient had a good quality of life with normal liver function until 3 months before death. Conclusions. This is the rst case report of LT as treatment of unresectable iASC using a marginal graft. -

Hubei WLAN Hotspot
Hubei WLAN hotspot NO. SSID Location_Name Location_Type Location_Address City Province 1 ChinaNet XiaoGan Hotel Hotel XiaoGan City Station Road, the middle XiaoGan 2 ChinaNet XiaoGan Dynasty Hotel Hotel XiaoGan City Station Road and Sports Road Interchange XiaoGan 3 ChinaNet XiaoGan JinYi Hotel Hotel XiaoGan City, the southern end of Railroad Station XiaoGan 4 ChinaNet XiaoGan Qiankun Business Hotel Hotel Qiankun XiaoGan City Avenue XiaoGan 5 ChinaNet XiaoGanRailroad Station Railroad Station XiaoGan City Railroad Station XiaoGan 6 ChinaNet XiaoGan Passenger station Bus Station XiaoGan City HuaiYing Avenue XiaoGan Zhong Bai 7 ChinaNet XiaoGan Huatai Securities Other XiaoGan city Long Road ZhongBai warehouse Side warehouse,Long Road, XiaoGan XiaoGan city 8 ChinaNet ZhongXiang Hotel Hotel ZhongXiangHotel ZhongXiang 9 ChinaNet JingBei Lake Grand Hotel Hotel gold Beihu Grand Hotel JingShan 10 ChinaNet Dynasty Hotel Hotel Dynasty Hotel ShaYang 11 ChinaNet JingMen International Hotel Hotel JingMen International Hotel JingMen 12 ChinaNet JingMen Phoenix Garden Hotel Hotel JingMen Phoenix Garden Hotel JingMen 13 ChinaNet Xiantao vocational colleges School Xiantao City, HongDa Road 118 XianTao 14 ChinaNet Days City Hotel Hotel Golden Road XianTao 15 ChinaNet Huanggang City Government Government Huanggang 71 Road HuangZhou 16 ChinaNet Huanggang Municipal Government Huanggang 71 Road HuangZhou 17 ChinaNet Huanggang the National People's Congress Government Huanggang 71 Road HuangZhou 18 ChinaNet Huanggang Telecom Company Telecom Huanggang test Street -

Modelling the Effects of Wuhan's Lockdown During COVID-19, China
Research Modelling the effects of Wuhan’s lockdown during COVID-19, China Zheming Yuan,a Yi Xiao,a Zhijun Dai,a Jianjun Huang,a Zhenhai Zhangb & Yuan Chenb Objective To design a simple model to assess the effectiveness of measures to prevent the spread of coronavirus disease 2019 (COVID-19) to different regions of mainland China. Methods We extracted data on population movements from an internet company data set and the numbers of confirmed cases of COVID-19 from government sources. On 23 January 2020 all travel in and out of the city of Wuhan was prohibited to control the spread of the disease. We modelled two key factors affecting the cumulative number of COVID-19 cases in regions outside Wuhan by 1 March 2020: (i) the total the number of people leaving Wuhan during 20–26 January 2020; and (ii) the number of seed cases from Wuhan before 19 January 2020, represented by the cumulative number of confirmed cases on 29 January 2020. We constructed a regression model to predict the cumulative number of cases in non-Wuhan regions in three assumed epidemic control scenarios. Findings Delaying the start date of control measures by only 3 days would have increased the estimated 30 699 confirmed cases of COVID-19 by 1 March 2020 in regions outside Wuhan by 34.6% (to 41 330 people). Advancing controls by 3 days would reduce infections by 30.8% (to 21 235 people) with basic control measures or 48.6% (to 15 796 people) with strict control measures. Based on standard residual values from the model, we were able to rank regions which were most effective in controlling the epidemic. -

Degree of Harmony of Urban Land Use and Economic Development: Case Study of Wuhan Metropolitan Area
Journal of Sustainable Development; Vol. 7, No. 4; 2014 ISSN 1913-9063 E-ISSN 1913-9071 Published by Canadian Center of Science and Education Degree of Harmony of Urban Land Use and Economic Development: Case Study of Wuhan Metropolitan Area Zhu Xiangbo1 & Tan Shukui1 1 College of Public Administration, Huazhong University of Science and Technology, Wuhan, China Correspondence: Tan Shukui, College of Public Administration, Huazhong University of Science and Technology, Wuhan, China. Tel: 86-27-8755-9342. E-mail:[email protected]; [email protected] Received: May 12, 2014 Accepted: May 27, 2014 Online Published: July 31, 2014 doi:10.5539/jsd.v7n4p139 URL: http://dx.doi.org/10.5539/jsd.v7n4p139 Abstract As urban land use is facing more and more severe sustainability challenges, it is essential to make effective and scientific evaluation on relationship between urban land use and economic development. With a series of indicators that drawn from previous literatures and data collected from Wuhan Metropolitan Area, this paper evaluated the degree of harmony, which characterizes the level of sustainable in the eight cities. By methods of cluster analysis and grey rational analysis, degree of harmony was categorized and calculated. Results revealed that harmony degree of different cities had certain gaps which were continually widening and can be divided into four categories. Relative scores on harmony of them are almost consistency with the category. It is found that the difference on land use put significant on the degree of harmony. Some other characteristics including city administrative status, comparative regional advantages also have a large impact. These findings lead further suggestions and recommendations for governments to design relevant policy.