Len Mckenzie Seagrass-Watch HQ
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Indigenous Women's Preferences for Climate Change Adaptation And
Indigenous women’s preferences for climate change adaptation and aquaculture development to build capacity in the Northern Territory Final Report Lisa Petheram, Ann Fleming, Natasha Stacey, and Anne Perry Indigenous women’s preferences for climate change adaptation and aquaculture development to build capacity in the Northern Territory Charles Darwin University Northern Territory Government Australian National University LISA PETHERAM, ANN FLEMING, NATASHA STACEY AND ANNE PERRY Published by the National Climate Change Adaptation Research Facility ISBN: 978-1-925039-55-9 NCCARF Publication 84/13 © 2013 Charles Darwin University This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the copyright holder. Please cite this report as: Petheram, L, Fleming, A, Stacey, N and Perry, A 2013 Indigenous women’s preferences for climate change adaptation and aquaculture development to build capacity in the Northern Territory, National Climate Change Adaptation Research Facility, Gold Coast, pp. 44. Acknowledgement This work was carried out with financial support from the Australian Government (Department of Climate Change and Energy Efficiency) and the National Climate Change Adaptation Research Facility. The views expressed herein are not necessarily the views of the Commonwealth or NCCARF, and neither the Commonwealth nor NCCARF accept responsibility for information or advice contained herein. The role of NCCARF is to lead the research community in a national interdisciplinary effort to generate the information needed by decision-makers in government, business and in vulnerable sectors and communities to manage the risk of climate change impacts. The authors would like to express their gratitude to the Warruwi community for their participation and support in this project. -

A Distinctive Voice in the Antipodes: Essays in Honour of Stephen A. Wild
ESSAYS IN HONOUR OF STEPHEN A. WILD Stephen A. Wild Source: Kim Woo, 2015 ESSAYS IN HONOUR OF STEPHEN A. WILD EDITED BY KIRSTY GILLESPIE, SALLY TRELOYN AND DON NILES Published by ANU Press The Australian National University Acton ACT 2601, Australia Email: [email protected] This title is also available online at press.anu.edu.au National Library of Australia Cataloguing-in-Publication entry Title: A distinctive voice in the antipodes : essays in honour of Stephen A. Wild / editors: Kirsty Gillespie ; Sally Treloyn ; Don Niles. ISBN: 9781760461119 (paperback) 9781760461126 (ebook) Subjects: Wild, Stephen. Essays. Festschriften. Music--Oceania. Dance--Oceania. Aboriginal Australian--Songs and music. Other Creators/Contributors: Gillespie, Kirsty, editor. Treloyn, Sally, editor. Niles, Don, editor. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the publisher. Cover design and layout by ANU Press. Cover photograph: ‘Stephen making a presentation to Anbarra people at a rom ceremony in Canberra, 1995’ (Australian Institute of Aboriginal and Torres Strait Islander Studies). This edition © 2017 ANU Press A publication of the International Council for Traditional Music Study Group on Music and Dance of Oceania. Aboriginal and Torres Strait Islander people are advised that this book contains images and names of deceased persons. Care should be taken while reading and viewing. Contents Acknowledgements . vii Foreword . xi Svanibor Pettan Preface . xv Brian Diettrich Stephen A . Wild: A Distinctive Voice in the Antipodes . 1 Kirsty Gillespie, Sally Treloyn, Kim Woo and Don Niles Festschrift Background and Contents . -

JERNUDD B01 Sound Recordings Collected by Bjorn H. Jernudd, 1967
Finding aid JERNUDD_B01 Sound recordings collected by Bjorn H. Jernudd, 1967 Prepared February 2012 by SL Last updated 6 December 2016 ACCESS Availability of copies Listening copies are available. Contact the AIATSIS Audiovisual Access Unit by completing an online enquiry form or phone (02) 6261 4212 to arrange an appointment to listen to the recordings or to order copies. Restrictions on listening Some materials in this collection are restricted and may only be listened to by those who have obtained permission from the relevant Indigenous individual, family or community. Refer to audition sheets below for more details. Restrictions on use This collection is open for copying to the relevant Indigenous individual, family or community. All other clients may only copy this collection with the permission of Bjorn Jernudd and the relevant Indigenous individual, family or community. Permission must be sought from Bjorn Jernudd and the relevant Indigenous individual, family or community for any publication or quotation of this material. Any publication or quotation must be consistent with the Copyright Act (1968). SCOPE AND CONTENT NOTE Date: 1967 Extent: 94 sound tape reels : analogue, mono ; 5 in. + field tape report sheets. Production history These recordings were collected between 28 October and December 1967 by linguist Bjorn Jernudd during fieldwork at Kulaluk / Bagot, Gunbalanya / Oenpelli and Barunga / Bamyili in the Northern Territory of Australia. The purpose of the field trips was to research the Djinang, Gunwinggu, Alawa, creoles and Aboriginal English of the Top End. Dr Jernudd also recorded meetings of the Bagot Council and conversations among the residents of Bagot and Gunbalanya. -

Threatened Plants and Animals in Kakadu National Park: a Review and Recommendations for Management
Threatened plants and animals in Kakadu National Park: a review and recommendations for management John Woinarski Project NHTKNP01 Report to Parks Australia North. September 2004. J.C.Z. Woinarski Northern Territory Department of Infrastructure Planning and Environment PO Box 496 Palmerston Northern Territory, 0831 Cover photograph: Arnhem rock-rat Zyzomys maini (photo: Greg Miles) SUMMARY This report comprises three main sections. The first section is an introduction that lists the currently recognised threatened plant and animal species that occur within Kakadu National Park; describes the process and criteria for listing; notes the substantial discrepancies in lists between the last comprehensive review of Kakadu’s threatened species (1995) and this report; and notes also the substantial discrepancy between national and Northern Territory listings for threatened species occurring in Kakadu. The second, and largest, section provides more specific information on each threatened species, noting in particular the status of each species within Kakadu National Park, as well as providing a broader conservation and management context. The third section collates information on management and threats across the set of threatened species, and draws research and management priorities for Kakadu National Park. The species occurring in Kakadu National Park that are listed as threatened under national and/or Northern Territory legislation are tabulated below. The current listing comprises a total of 16 plant species (of which 6 are listed at national level) and 31 animal species (of which 16 are listed at national level). An additional plant species has been nominated to be added at the next revision of the NT list. Information is also presented on four threatened plant species with records from near Kakadu, and considered reasonably likely to occur within Kakadu. -

The Mamurrng Ceremony of Western Arnhem Land Reuben Brown
1 A Different Mode of Exchange: The Mamurrng Ceremony of Western Arnhem Land Reuben Brown A sensory overload ‘Hold on!’ I shout to my partner Rachel and yabok (K: sister)1 Rhonda, as we hit a wave that almost sends us flying over the side of our tin boat. We grip the edge of the boat tightly, and I check to see whether the recording gear at my feet is still dry. We are now completely at the whim of the ocean as our boat skips along the waves, tracking from Sandy Creek, on the mainland of Arnhem Land in the north coast of Australia, to Warruwi, Goulburn Island—where the Mamurrng (diplomacy or exchange ceremony; see Berndt 1951: 156) will take place. Back in the Aboriginal community of Gunbalanya, where my adopted family reside and where we began our journey, the surrounding rocks of the Arnhem Land plateau are baking and the wetlands drying out to a crust, as the hot weather of the dry season continues to scorch the earth, with no rain in sight. But out here, the wind and spray of the ocean provides welcome 1 Throughout this article, significant words relating to western Arnhem Land songs in the Aboriginal languages of Kunwinjku (K) and Mawng (M) are indicated in italics (except for proper nouns such as the names of people and song-sets). English translations are glossed in brackets the first time the word appears in the text. I adopt standard orthographies for both Kunwinjku and Mawng. 41 A DISTINCTIVE VOICE IN ThE ANTIPODES relief from the harsh build-up of heat. -

10 Gapu Dhulway, Gapu Maramba: Conceptualisation and Ownership of Saltwater Among the Burarra and Yan- Nhangu Peoples of Northeast Arnhem Land
10 Gapu Dhulway, Gapu Maramba: conceptualisation and ownership of saltwater among the Burarra and Yan- nhangu peoples of northeast Arnhem Land Geoffrey Bagshaw Although a considerable body of literature now exists on aspects of cus- tomary marine tenure and/resource usage in the Blyth River—Crocodile Islands region of northeast Arnhem Land,1 little has been written about the ways in which the local Burarra and Yan-nhangu peoples actually conceptualise saltwater and articulate concomitant relations of sea ownership. Ultimately, and perhaps inevitably, this lack of focus upon indigenous conceptions of saltwater has resulted in the promulgation and uncritical acceptance of certain ethnographically inaccurate rep- resentations of regional sea tenure patterns and principles. Foremost among these (mis)representations is the widely held view that tradi- tional patterns of land and sea tenure in this region are, to all practical intents and purposes, identical (cf. Davis 1984a; Davis and Prescott 1992; Cooke 1995:9–10; Keen 1984–85:433; see also Northern Land Council 1992:1, 1995:2,4–5). Deriving from this perspective are the equally erroneous, if often less explicitly articulated, assumptions to the 1 See, for example, Aboriginal Land Commissioner (1981); Bagshaw (1979, 1991a, 1991b, 1991c, 1995a); Cooke (n.d.[a], n.d.[b], 1995); Davis (1981, 1984a, 1984b); Davis and Prescott (1992); Dreyfus and Dhulumburrk (1980); Keen (1977, 1981, 1984–85); Meehan (1977a, 1977b, 1977c, 1982, 1983); and Ritchie (1987). Similar material from neighbouring parts of Arnhem Land can be found in Aboriginal Land Commissioner (1988); Davis (1981, 1982); Ginytjirrang Mala/ADVYZ (1994); Hiatt (1982); Keen (1982, 1983); Northern Land Council (1992, 1995); and Palmer (1984–85). -

Macassan History and Heritage Journeys, Encounters and Influences
Macassan History and Heritage Journeys, Encounters and Influences Edited by Marshall Clark and Sally K. May Macassan History and Heritage Journeys, Encounters and Influences Edited by Marshall Clark and Sally K. May Published by ANU E Press The Australian National University Canberra ACT 0200, Australia Email: [email protected] This title is also available online at http://epress.anu.edu.au National Library of Australia Cataloguing-in-Publication entry Author: Clark, Marshall Alexander, author. Title: Macassan history and heritage : journeys, encounters and influences / Marshall Clark and Sally K. May. ISBN: 9781922144966 (paperback) 9781922144973 (ebook) Notes: Includes bibliographical references. Subjects: Makasar (Indonesian people)--Australia. Northern--History. Fishers--Indonesia--History Aboriginal Australians--Australia, Northern--Foreign influences. Aboriginal Australians--History. Australia--Discovery and exploration. Other Authors/Contributors: May, Sally K., author. Dewey Number: 303.482 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the publisher. Cover images: Fishing praus and cured trepang in the Spermonde Archipelago, South Sulawesi. Source: Marshall Clark. Cover design and layout by ANU E Press Printed by Griffin Press This edition © 2013 ANU E Press Contents 1. Understanding the Macassans: A regional approach .........1 Marshall Clark and Sally K. May 2. Studying trepangers. 19 Campbell Macknight 3. Crossing the great divide: Australia and eastern Indonesia ... 41 Anthony Reid 4. Histories with traction: Macassan contact in the framework of Muslim Australian history ....................... 55 Regina Ganter 5. Interpreting the Macassans: Language exchange in historical encounters .................................. -

Living on Saltwater Country Saltwater on Living Background
Living on Saltwater Country Background P APER interests in northernReview Australianof literature marine about environments Aboriginal rights, use, management and Living on Saltwater Country Review of literature about Aboriginal rights, use, ∨ management and interests in northern Australian marine environments Healthy oceans: cared for, understood and used wisely for the Healthy wisely benefit of all, now and in the future. Healthy oceans: cared for, for the Healthy understood and used wisely for the benefit of all, oceans: for the National Oceans Office National Oceans Office National Oceans Office Level 1, 80 Elizabeth St, Hobart GPO Box 2139, Hobart, TAS, Australia 7001 Tel: +61 3 6221 5000 Fax: +61 3 6221 5050 www.oceans.gov.au The National Oceans Office is an Executive Agency of the Australian Government Living on Saltwater Country Aboriginal and Torres Strait Islander readers should be warned that this document may contain images of and quotes from deceased persons. Title: Living on Saltwater Country. Review of literature about Aboriginal rights, use, management and interests in northern Australian marine environments. Copyright: © National Oceans Office 2004 Disclaimers: General This paper is not intended to be used or relied upon for any purpose other than to inform the management of marine resources. The Traditional Owners and native title holders of the regions discussed in this report have not had the opportunity for comment on this document and it is not intended to have any bearing on their individual or group rights, but rather to provide an overview of the use and management of marine resources in the Northern Planning Area for the Northern regional marine planning process. -

List of Northern Territory Birds
WESTERN AUSTRALIAN MUSEUM SPECIAL PUBLICA TIO N No. 4 LIST OF NORTHERN TERRITORY BIRDS World List Abbreviation : Spec. Publ. W. Aust. Mus. Printed for the Western Australian Museum Board by the Government Printer, Perth, Western Austral,ia. Iss~ecl 28th February, 1967 Edited by w. D. L. RIDE and A. NEUMANN RED- WINGED PARROT Photograph by Mr. Peter Slater LIST OF NORTHERN TERRITORY BIRDS BY G. M. STORR [2]-13314 Table of Contents INTRODUCTION 7 Classification ... 7 Distribution ... 8 Status, habitat and breeding season 8 Appendices 9 LIST OF BIRDS 11 BIBLIOGRAPHY 65 SPECIES CONFIRMANDAE .... 69 GAZETTEER .... 71 INDEX TO SPECIES .. 84 Introduction It has long been the desire of ornithologists to have a list of Australian birds with their known range set out with considerably more precision than in current checklists. Yet it is hard to see how such a list can be compiled until each of the states and territories has a list of its own. Several state lists have appeared in the last two decades, and the only large gaps remaining are the birds of Queensland and the Northern Territory. In choosing the second as my subject, I have undertaken much the lighter task; for the avi fauna of the Territory is impoverished compared to Queensland's, and its literature is far smaller. The present work is a compendium of what has been published on the occurrence, status, habitat and breeding season of Northern Territory birds, augmented with my own field notes and those of Dr. D. L. Serventy. No attempt has been made to fill the numerous gaps in the record by writing to museums and observers for information or by personally exploring un worked regions. -
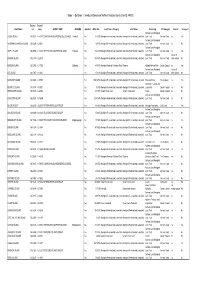
Stage 1 - Database 1: Introduced Species on Northern Territory Islands (Com ID: 49530)
Stage 1 - Database 1: Introduced Species on Northern Territory Islands (Com ID: 49530) Decimal Decimal Island Name Lat Long GROUP_NAME INDNAME Gazetteer AREA_HA_ Land Tenure Category Land Status Ownership NT Bioregion Joined? Surveyed? Arnhem Land Aboriginal ALGER ISLAND 135.96611 -11.87810 CUNNINGHAM ISLANDS/WESSEL ISLANDS Toombuli Yes 845.333 Aboriginal Freehold and Leasehold Aboriginal Freehold and Leasehold Land Trust Arnhem Coast no Yes Arnhem Land Aboriginal ANGARMBULUMARDJA ISLAND 136.63495 -13.66611 Yes 169.956 Aboriginal Freehold and Leasehold Aboriginal Freehold and Leasehold Land Trust Arnhem Coast no No Arnhem Land Aboriginal ASTELL ISLAND 136.39856 -11.88248 THE ENGLISH COMPANYS ISLANDS Yinbadak Yes 1264.439 Aboriginal Freehold and Leasehold Aboriginal Freehold and Leasehold Land Trust Arnhem Coast no Yes Arnhem Land Aboriginal not an off BANYAN ISLAND 135.21349 -12.25819 Yes 4943.473 Aboriginal Freehold and Leasehold Aboriginal Freehold and Leasehold Land Trust Arnhem Coast shore island No BARRON ISLAND 132.35042 -12.17392 Djidbordu Yes 450.472 Aboriginal Freehold - National Park Reserve Kakadu National Park Darwin Coastal no Yes Arnhem Land Aboriginal not an off BAT ISLAND 134.17367 -12.10151 Yes 376.139 Aboriginal Freehold and Leasehold Aboriginal Freehold and Leasehold Land Trust Arnhem Coast shore island No BATHURST ISLAND 130.32948 -11.57578 Yes 169319.353 Aboriginal Freehold and Leasehold Aboriginal Freehold and Leasehold Tiwi Land Trust Tiwi Cobourg no Yes Aboriginal Freehold and BEATRICE ISLAND 135.78374 -15.09723 -

Picturesque Atlas of Australasia Maps
A-Signal Battery. I-Workshops. B-Observatory . K-Government House. C-Hospital. L-Palmer's Farm. .__4 S URVEY D-Prison. M-Officers ' Quarters. of E-Barracks . N-Magazine. F-Store Houses. 0-Gallows. THE SET TLEMENT ;n i Vh u/ ,S OUTN ALES G-Marine Barracks . P-Brick-kilns. H-Prisoners ' Huts. Q-Brickfields. LW OLLANI) iz /` 5Mile t4 2 d2 36 Engraved by A.Dulon 4 L.Poates • 1FTTh T i1111Tm»iTIT1 149 .Bogga 1 a 151 Bengalla • . l v' r-- Cootamundra Coola i r A aloe a 11lichellago 4 I A.J. SCALLY DEL. , it 153 'Greggreg ll tai III IJL. INDEX TO GENERAL MAP OF NE W SOUTH W ALES . NOTE -The letters after the names correspond with those in the borders of the map, and indicate the square in which the name will be found. Abercrombie River . Billagoe Mountain Bundella . J d Conjurong Lake . Dromedary Mountain. Aberdeen . Binalong . Bunda Lake C d Coogee . Drummond Mountain. Aberfoyle River . Binda . Bundarra . L c Cook (county) . Dry Bogan (creek) Acacia Creek . Bingera . Bunganbil Hill G g Coolabah . Dry Lake . Acres Billabong . Binyah . Bungarry Lake . E g Coolaburrag u ndy River Dry Lake Adelong Bird Island Bungendore J h Coolac Dry Lake Beds . Adelong Middle . Birie River Bungle Gully I c Coolah . Dry River . Ailsa . Bishop 's Bridge . Bungonia . J g Coolaman . Dubbo Creek Albemarle Black Head Bunker 's Creek . D d Coolbaggie Creek Dubbo Albert Lake . Blackheath Bunna Bunna Creek J b Cooleba Creek Duck Creek Albury . Black Point Bunyan J i Cooma Dudanman Hill . Alice Black Swamp Burbar Creek G b Coomba Lake Dudley (county) . -

A Survey of Intertidal Seagrass from Van Diemen Gulf to Castlereagh Bay, Northern Territory, and from Gove to Horn Island, Queensland
A survey of intertidal seagrass from Van Diemen Gulf to Castlereagh Bay, Northern Territory, and from Gove to Horn Island, Queensland Report to the National Oceans Office Department of Primary Industries & Fisheries 1, CRC Reef Research Centre Biodiversity and Conservation, NT Department of Infrastructure, Planning and Environment 2 Anthony Roelofs 1, Rob Coles 1 and Neil Smit 2 25 March 2005 Acknowledgments This project was funded by the National Oceans Office with support from the Natural Heritage Trust (Australian Government Department of Environment and Heritage), Northern Territory Department of Infrastructure, Planning and Environment, Parks Australia North, and the Queensland Department of Primary Industries and Fisheries. The Marine Ecology Group is partially funded by CRC Reef Research Centre. The authors wish to thank the following people: the Traditional Owners for granting access to the land and sea country surveyed in this report; National Oceans Office Ocean Liaison Officers Chantal Roder and Ilse Kiessling for Indigenous community consultation and distributing information flyers before the survey; Neil Dodds (pilot) and Yvonne Wallace (manager) of Cape York Helicopters for assistance with planning and the smooth conduct of a difficult logistic exercise; Len McKenzie (QDPI&F) and the National Oceans Office for assistance with the text; and NT Parks rangers Phil Wise (Gove) and David Wurst (Boroloola) for their support during the survey. We also wish to acknowledge Terry Mahney (NLC) for guiding and assisting in the consultation process and we would also like to thank in particular: Freddy Gabiya, Solomon Garnuwa, Jim Gorey and Ronald Lamilami, the Mardbalk Rangers and the Warruwi Community Council (Warrawi); Alistair James, Don Wilton, Ray Hall, Stuart Ankin, Djelk Rangers and Bawinanga Aboriginal Corporation (Maningrida); Bentley James, Murrunga Island residents, Jeffrey Malawa and Milingimbi Community Council (Millingimbi); and Solomon Ryan, Charlie Ramandjarri, Ramingining Homelands Resource Centre Inc and Wanga Djakimirr Rangers.