Marine Science for High School Students in Chemistry, Biology And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Our Tobacco-Free Resource Guide for Youth
educate. engage. empower. TOBACCO-FREE COMMUNITY FORUM TOBACCO-FREE RESOURCE GUIDE for Youth, Communities, Parents, and Schools TOBACCO-FREE COMMUNITY FORUM Educate. Engage. Empower. April 28, 2021 This event made possible by the Poe Center for Health Education in partnership with Wake County Human Services, Wake County Public Schools, and the American Heart Association . TOBACCO-FREE COMMUNITY FORUM AGENDA 9:15 a.m. - 9:30 a.m. - Log-In and Sign-On Slideshow of PhotoVoice Projects 9:30 a.m. - 9:40 a.m. - Welcome & Opening Remarks Honorable Sig Hutchinson, Wake County Commissioner Ann Rollins, Executive Director, Poe Center For Health Education Roxie Cash, Wake County Board of Education 9:40 a.m. - 9:45 a.m. - Recognition of Local Champions Presented by Youth Empowerment Team 9:45 a.m. - 10:00 a.m. - A Snapshot of Tobacco’s Impact on Youth In Wake County Sumedh Kotrannavar, Student at Carnage Middle School Teja Wasudev, Student at Cary Academy Nikhil Patel, Student at Enloe High School Gorja Yadav, Student at Enloe High School Lily Zahn, Student at Fuquay-Varina High School 10:00 a.m. - 10:20 a.m. - Latest Updates on Tobacco Impacts, Trends, and Statistics Jim Martin, Director of Policy and Programs with the N.C. Tobacco Prevention and Control Branch Division of Public Health, NC Department of Health and Human Services 10:20 a.m. - 10:45 a.m. - Youth Perspective on Local Tobacco Policy Needs Panel Moderator: Honorable Sig Hutchinson, Wake County Commissioner Panelists: Sumedh Kotrannavar, Student at Carnage Middle School Teja Wasudev, Student at Cary Academy Nikhil Patel, Student at Enloe High School Lily Zahn, Student at Fuquay-Varina High School 10:45 a.m. -

NYLES I. FLEMING Email: [email protected] 218 Fox Walk Path Garner, NC 27529 Cell: (919) 539-5696
NYLES I. FLEMING Email: [email protected] 218 Fox Walk Path Garner, NC 27529 Cell: (919) 539-5696 EDUCATION Morehouse College, Atlanta, Georgia June 2020 (expected) Applied Physics and Environmental Engineering Physics and Dual Degree Engineering Program (DDEP) Academic Scholarship GPA: 3.3 Credit Hours: 42 AUC Dual Degree Engineering Program Honor Student Dual Degree Engineering Program Shining Star Award/ Scholarship Recipient William G. Enloe High School, Raleigh, North Carolina June 2015 GPA: 3.5 ACTIVITIES Vice President of Student Organization for Sustainability (S.O.S): 2016–Present Studied abroad in Beijing China: May 2016 - June 2016 Alpha Lambda Delta Honors Society Member: 2016–Present Applied Engineering Response Organization (AERO): 2015–Present National Society of Black Engineers Jr (NSBE): 2014–Present Peer Discovery Program (special education department and Special Olympics volunteer): 2014–2015 “Students With A Goal” Leadership Program (Mentor): 2011–2015 100 Black Men of America Leadership Program: 2011–2015 Participated in research project at the Biogen Idec Community Lab: 2014 Delta Carousel Leadership Academy: 2002–2015 VOLUNTEER EXPERIENCE Maintain community garden in West End community through student organized club (S.O.S): 2016–Present Missionary in Ghana, West Africa: Summer 2014 Church building project – Assisted construction workers with heavy tasks such as operating concrete mixer and placement of cement blocks and pipes for a church extension Youth Ambassador – Visited schools, mentored -

NGPF's 2021 State of Financial Education Report
11 ++ 2020-2021 $$ xx %% NGPF’s 2021 State of Financial == Education Report ¢¢ Who Has Access to Financial Education in America Today? In the 2020-2021 school year, nearly 7 out of 10 students across U.S. high schools had access to a standalone Personal Finance course. 2.4M (1 in 5 U.S. high school students) were guaranteed to take the course prior to graduation. GOLD STANDARD GOLD STANDARD (NATIONWIDE) (OUTSIDE GUARANTEE STATES)* In public U.S. high schools, In public U.S. high schools, 1 IN 5 1 IN 9 $$ students were guaranteed to take a students were guaranteed to take a W-4 standalone Personal Finance course standalone Personal Finance course W-4 prior to graduation. prior to graduation. STATE POLICY IMPACTS NATIONWIDE ACCESS (GOLD + SILVER STANDARD) Currently, In public U.S. high schools, = 7 IN = 7 10 states have or are implementing statewide guarantees for a standalone students have access to or are ¢ guaranteed to take a standalone ¢ Personal Finance course for all high school students. North Carolina and Mississippi Personal Finance course prior are currently implementing. to graduation. How states are guaranteeing Personal Finance for their students: In 2018, the Mississippi Department of Education Signed in 2018, North Carolina’s legislation echoes created a 1-year College & Career Readiness (CCR) neighboring state Virginia’s, by which all students take Course for the entering freshman class of the one semester of Economics and one semester of 2018-2019 school year. The course combines Personal Finance. All North Carolina high school one semester of career exploration and college students, beginning with the graduating class of 2024, transition preparation with one semester of will take a 1-year Economics and Personal Finance Personal Finance. -

Enloe Junior's Tech Company Takes
May 2015 VOLUME XXXIII ISSUE VI WILLIAM G. ENLOE HIGH SCHOOL http://enloenews.kinja.com Enloe IN NEWS Enloe junior’s tech Google Fiber hits embraces the Triangle The brand new internet ser- company takes off Day of vice from Google has made a home in Raleigh. Silence By Caroline Rexrode IN OPINION On Friday, April 17th, hun- The Good Friday dreds of Enloe students par- ticipated in the Day of Silence, controversy successfully showing solidar- Wake County Schools fl ip- ity with LGBTQ students and fl ops on Good Friday can- spreading awareness for a cellation. global cause. According to the Enloe GSA co-presidents Brennan Lewis and Sophie Crouse, the ultimate goal of the Day of Silence is to make LGBT stu- IN FEATURES dents feel safe, supported and represented, as well as pro- Foodie Flavors in voke people to consider the silencing of LGBTQ students East Raleigh in schools due to bullying, ha- Explore new and exciting op- rassment and lack of support Courtesy of Neil Peterson tions for off-campus eats, right from teachers. in Enloe’s backyard. Entrepreneur and founder of Linker Logic Ritwick Pavan will soon start development of Enloe’s GSA encourages its WRAL’s new Apple Watch application. members and all willing stu- dents to participate in the Day By Jacob Sichel of Silence to raise awareness of At fi rst glance, eleventh junior-year schedule. He takes “everyone at Enloe is always the censoring of identity that grader Ritwik Pavan seems like six AP classes without lunch, doing something diff erent. -
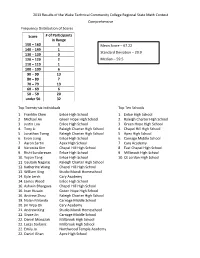
2013Results.Pdf
2013 Results of the Wake Technical Community College Regional State Math Contest Comprehensive Frequency Distribution of Scores Score # of Participants in Range 150 – 160 3 Mean Score – 67.22 140 – 149 1 Standard Deviation – 29.9 130 – 139 0 120 – 129 2 Median – 59.5 110 – 119 1 100 – 109 6 90 – 99 13 80 – 89 7 70 – 79 13 60 – 69 6 50 – 59 20 under 50 32 Top Twenty-six Individuals Top Ten Schools 1. Franklin Chen Enloe High School 1. Enloe High School 2. Michael An Green Hope High School 2. Raleigh Charter High School 3. Justin Luo Enloe High School 3. Green Hope High School 4. Tony Li Raleigh Charter High School 4. Chapel Hill High School 5. Jonathan Tseng Raleigh Charter High School 5. Apex High School 6. Evan Liang Enloe High School 6. Carnage Middle School 7. Aaron Sartin Apex High School 7. Cary Academy 8. Veronica Kim Chapel Hill High School 8. East Chapel High School 9. Rishi Sundaresan Enloe High School 9. Millbrook High School 10. Yujian Tang Enloe High School 10. CE Jordan High School 11. Gautam Nagaraj Raleigh Charter High School 12. Katherine Wang Chapel Hill High School 13. William King Studio Mundi Homeschool 14. Kyle Lerch Cary Academy 14. James Wood Enloe High School 16. Ashwin Bhargava Chapel Hill High School 16. Inan Husain Green Hope High School 16. Andrew Zhou Raleigh Charter High School 19. Nolan Miranda Carnage Middle School 20. Jin Woo Ok Cary Academy 21. Andrew King Studio Mundi Homeschool 22. Grace Jin Carnage Middle School 22. -

WAKE COUNTY BOARD of EDUCATION MEETING MINUTES October 21, 2008
WAKE COUNTY BOARD OF EDUCATION MEETING MINUTES October 21, 2008 Board Members Present Staff Members Present Rosa Gill, Chair Superintendent Del Burns Donna Hargens Kevin L. Hill, Vice Chair Terri Cobb Don Haydon Beverley Clark Danny Barnes David Holdzkom Eleanor Goettee Mike Burriss Ann Hooker Patti Head Kathy Chontos Bev White Anne McLaurin Marvin Connelly Jonibel Willis Ron Margiotta Chuck Dulaney Mark Winters Lori Millberg Michael Evans Board Attorney Present Horace Tart Lloyd Gardner Ann Majestic Chair Rosa Gill called the meeting to order at 3:04 p.m. Everyone recited the Pledge of Allegiance. Chair’s Comments • Ms. Gill congratulated Principal of the Year, Matt Wight, principal at Apex High School and the WCPSS 2008 Assistant Principal of the Year, Shejuanna Rodgers, assistant principal of Apex Middle School. Ms. Gill thanked all of the principals and assistant principals for their hard work and dedication they give to the schools and students of Wake County. • On October 16, the ribbon-cutting for Lynn Road Elementary School was held. Ms. Gill provided a welcome from the Board of Education and Ms. Clark was in attendance. • On Friday, October 17, Ms. Gill attended the dedication of the Shaw University Center for Early Childhood Education, Development and Research. • On October 9, Ms. Gill attended the Freedom Fund Banquet sponsored by the State NAACP. • Ms. Gill shared that over the past month the Board has met with mayors and municipality boards. Meetings were held on September 22 with the Towns of Knightdale, Zebulon, and Raleigh, September 25 with the Towns of Apex, Morrisville, Holly Springs, and Fuquay-Varina, September 30, with the Towns of Wake Forest, Rolesville, and Wendell, and October 14, with the Towns of Cary and Garner. -
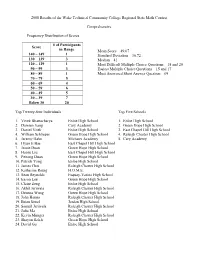
2008Results.Pdf
2008 Results of the Wake Technical Community College Regional State Math Contest Comprehensive Frequency Distribution of Scores # of Participants Score in Range Mean Score – 49.07 140 – 149 1 Standard Deviation – 36.72 130 – 139 3 Median – 41 120 – 129 1 Most Difficult Multiple Choice Questions – 18 and 20 90 – 99 3 Easiest Multiple Choice Questions – 15 and 17 80 – 89 1 Most Answered Short Answer Question – 69 70 – 79 5 60 – 69 4 50 – 59 6 40 – 49 5 30 – 39 7 Below 30 20 Top Twenty-four Individuals Top Five Schools 1. Vivek Bhattacharya Enloe High School 1. Enloe High School 2. Damien Jiang Cary Academy 2. Green Hope High School 3. Daniel Vitek Enloe High School 3. East Chapel Hill High School 4. William Schlieper Green Hope High School 4. Raleigh Charter High School 5. Jeremy Hahn Michaux Academy 5. Cary Academy 6. Hyun Ji Bae East Chapel Hill High School 7. Jessie Duan Green Hope High School 8. Haoru Liu East Chapel Hill High School 9. Peitong Duan Green Hope High School 10. Patrick Yang Enloe High School 11. James Choi Raleigh Charter High School 12. Katherine Rettig H.O.M.E. 13. Sean Reynolds Fuquay-Varina High School 14. Eason Lee Green Hope High School 15. Claire Zeng Enloe High School 16. Akhil Jariwala Raleigh Charter High School 17. Deanna Wung Green Hope High School 18. John Hanna Raleigh Charter High School 19. Brian Simel Jordan High School 20. Saumil Jariwala Raleigh Charter High School 21. Julia Ma Enloe High School 22. Kevin Munger Raleigh Charter High School 23. -

WCPSS 2021-2022 High School Program Planning Guide
High School Program Planning Guide 2021-2022 1/8/2021 Table of Contents 3 General Information 3 Graduation Requirements 4 Endorsements 6 Graduation Requirements Chart 7 Scheduling High School Courses in Middle School 9 University of North Carolina: Minimum Admission Requirements 10 Promotion Requirements 11 Course Requirements: Course Loads, Course Selection, & Course Withdrawal 11 Grades, Class Rank & Honors 13 Transfer Credit 13 Transcripts 14 Graduation: Early Graduation, Mid-Year Graduation 15 Program Details: Drivers Education, NCAA Eligibility, Exceptional Students, Study Abroad 16 Program Details: NC Virtual Public School, Credit Recovery 17 Alternative Programs of Study: AIG, Advanced Placement, Dual Enrollment 18 Application High Schools 22 Course Details 22 Arts Education 28 Career & Technical Education 94 English Language Arts Courses 99 English as a Second Language Courses 100 Healthful Living Courses 103 JROTC Courses 108 Mathematics Courses 112 Science Courses 115 Social Studies Courses 118 Special Education Courses 123 World Language Courses 126 Other Credit Programs 127 High School Course Codes In compliance with federal law, Wake County Public School System administers all education programs, employment activities, and admissions without discrimination against any person on the basis of gender, race, color, religion, national origin, age or disability. If you have questions or concerns please visit the following site for further information: https://www.wcpss.net/non-disc-policy High School Program Planning Guide 2021-2022 1 Welcome to that exciting time of year when you choose the courses you will take during the upcoming school year. The Wake County Public School System’s high school program provides students many options based on their career goals, needs, and individual interests. -

WAKE COUNTY PUBLIC SCHOOL SYSTEM 2016-2017 Freshman Planning Guide 1
WAKE COUNTY PUBLIC SCHOOL SYSTEM 2016-2017 Freshman Planning Guide 1 Welcome to High School! Dear Student, You are about to begin four of the most memorable years of your life. We want to make sure these memories are filled with an abundance of learning, life-long friendships, personal growth, career knowledge, and goal setting. As you begin your first year in high school, please remember that you have a support system of counselors, teachers, and administrators to help you make great strides, find success in the most difficult tasks, and enjoy this wonderful experience! School counselors will be available to assist you with academic and personal issues as well as to address your concerns about life after high school. During the school year, they will visit your classrooms and work with you individually or in groups. We hope to make your transition to high school a smooth and enjoyable one. This Freshman Planning Guide has been designed especially for you – The Class of 2020. It contains information essential for you to know as a new high school student in the Wake County Public School System. Read it, take notes, highlight important details, complete the fun activities, and refer to it throughout the school year. We wish you the best of luck! Student Services Staff 2 Table of Contents I. ACADEMICS - All About the Grade 4 The Block Schedule 2016-2017 5 High School General Information 6 Future Ready Core Graduation Requirements 10 End-of-Course Tests & VOCATS 11 Promotion to the 10th Grade 11 Attendance 12 Transcript Example 13 Translating your Transcript 14 Calculating your GPA 15 UNC System Admission Requirements 16 Information for Undocumented Students 17 NCAA Eligibility Requirements 18 II. -

Educational Directory of North Carolina
c.z NORTH CAROLINA EDUCATIONAL DIRECTORY 1907 - 1908 f<r' A iV^rRV.^ f O < z o o o CO < 2 (a o Q c o X -c UJ Z NORTH CAROLINA EDUCATIONAL DIRECTORY 1967-68 Issued by State Department of Public Instruction, Raleigh Publication No. 407 CONTENTS Page State Board of Education 3 State Department of Public Instruction 3 Controller's Office 17 Department of Community Colleges 20 State Board of Higher Education 21 State Education Assistance Authority 22 State Textbook Commission 22 State Evaluation Committee on Teacher Education 22 Teachers' and State Employees' Retirement System 23 North Carolina Education Association 23 North Carolina Teachers Association 24 North Carolina Congress of Colored Parents and Teachers 26 North Carolina Congress of Parents and Teachers 26 North Carolina Association for Childhood Education 26 North Carolina State School Boards Association 26 United Forces for Education 26 North Carolina Association of Colleges and Universities 26 Association for School, College and University Staffing in N. C. 27 Educational Periodicals 27 The Governor's School of North Carolina 28 North Carolina School of the Arts 28 The North Carolina Advancement School 29 The North Carolina Fund 29 The Learning Institute of North Carolina (LINC) 29 Institutions Providing Education Beyond the High School 30 Book, Map and Globe Representatives 36 Audiovisual Dealers Association 39 State and Territorial Superintendents 40 State Schools for the Handicapped 41 County and City School Administrative Units 42 Federal Schools 108 Approved Nonpublic Schools, 1966-67 110 Code Numbers for School Administrative Units 114 Alphabetical Index—North Carolina Public Schools 115 State Offices 3 STATE BOARD OF EDUCATION Dist. -
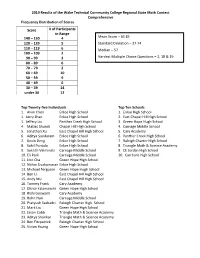
2019 Results of the Wake Technical Community College Regional State Math Contest Comprehensive Frequency Distribution of Scores
2019 Results of the Wake Technical Community College Regional State Math Contest Comprehensive Frequency Distribution of Scores Score # of Participants in Range 140 – 150 4 Mean Score – 65.85 120 – 139 5 Standard Deviation – 37.74 110 – 119 6 Median – 57 100 – 109 2 90 – 99 2 Hardest Multiple Choice Questions – 2, 18 & 19 80 – 89 6 70 – 79 2 60 – 69 10 50 – 59 9 40 – 49 6 30 – 39 14 under 30 12 Top Twenty-five Individuals Top Ten Schools 1. Alvin Chen Enloe High School 1. Enloe High School 1. Jerry Zhao Enloe High School 2. East Chapel Hill High School 1. Jeffery Liu Panther Creek High School 3. Green Hope Hugh School 4. Matias Shundi Chapel Hill High School 4. Carnage Middle School 5. Jonathan Xu East Chapel Hill High School 5. Cary Academy 6. Aditya Sundaram Enloe High School 6. Panther Creek High School 7. Kevin Deng Enloe High School 7. Raleigh Charter High School 8. Sahil Pontula Enloe High School 8. Triangle Math & Science Academy 9. Sukrith Velmineti Carnage Middle School 9. CE Jordan High School 10. Eli Park Carnage Middle School 10. Carrboro High School 11. Jiho Cha Green Hope High School 12. Nithin Sivakumaran Enloe High School 13. Michael Ferguson Green Hope Hugh School 14. Ben Li East Chapel Hill High School 15. Andy Mu East Chapel Hill High School 16. Tommy Frank Cary Academy 17. Dhruv Yalamanchi Green Hope High School 18. Rishi Goswami Cary Academy 19. Rohit Hari Carnage Middle School 20. Pratyush Seshadri Raleigh Charter High School 21. Mark Liu Green Hope High School 22. -

Prepárese Para Los Desastres
PREPÁRESE PARA LOS DESASTRES ¡Mantenga esta guía a la mano en caso de una emergencia! @readywake readywake.com 919-856-6480 La Administración de Emergencias del Condado Wake ha desarrollado el programa WakeListo (ReadyWake) para ayudarle a usted y a su familia a estar preparados en caso PROTECCIÓN EN CASA O EL TRABAJO de desastres. Utilice la información en este folleto para aprender a estar mejor preparado ante una emergencia. ENTRE Y QUÉDESE ADENTRO En algunas emergencias es más seguro quedarse en su sitio, bajo techo, fuera de carreteras y alejado de multitiudes. Principalmente durante incidentes que involucren a las fuerzas del orden debido a una amenaza latente para la comunidad, pero también durante otras emergencias, es posible que las autoridades locales le indiquen entrar y quedarse adentro. Usted ¡PREPÁRESE! LISTA DEL KIT PARA EMERGENCIAS: deberá permenecer dentro de su casa o lugar de trabajo y esperar a que las autoridades locales indiquen que la situación ha sido resuelta. Deberá monitorear la televisión, radio o fuentes locales de Internet confiables para recibir instrucciones. Tenga listo un plan para cuando deba abandonar su hogar. Artículos para familiares con Decida cómo su familia abandonará su hogar en caso de necesidades especiales y mascotas, emergencia. Seleccione un lugar seguro para encontrarse fuera de como medicamentos recetados, gafas, REFUGIARSE EN SU SITIO su casa. Practiquen su plan de evacuación. Adquiera combustible solución para lentes de contacto, para generadores y vehículos. baterías para auxiliares auditivos y medio Durante algunas emergencias, como emergencias químicas o radiológicas, es posible que se le pida refugiarse en su sitio.