Mechanical Characterization of the Interspinous Ligament Using Anisotropic Small Punch Testing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synovial Joints Permit Movements of the Skeleton
8 Joints Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Joint Structure and Movement Learning Outcomes 8.1 Contrast the major categories of joints, and explain the relationship between structure and function for each category. 8.2 Describe the basic structure of a synovial joint, and describe common accessory structures and their functions. 8.3 Describe how the anatomical and functional properties of synovial joints permit movements of the skeleton. © 2018 Pearson Education, Inc. Section 1: Joint Structure and Movement Learning Outcomes (continued) 8.4 Describe flexion/extension, abduction/ adduction, and circumduction movements of the skeleton. 8.5 Describe rotational and special movements of the skeleton. © 2018 Pearson Education, Inc. Module 8.1: Joints are classified according to structure and movement Joints, or articulations . Locations where two or more bones meet . Only points at which movements of bones can occur • Joints allow mobility while preserving bone strength • Amount of movement allowed is determined by anatomical structure . Categorized • Functionally by amount of motion allowed, or range of motion (ROM) • Structurally by anatomical organization © 2018 Pearson Education, Inc. Module 8.1: Joint classification Functional classification of joints . Synarthrosis (syn-, together + arthrosis, joint) • No movement allowed • Extremely strong . Amphiarthrosis (amphi-, on both sides) • Little movement allowed (more than synarthrosis) • Much stronger than diarthrosis • Articulating bones connected by collagen fibers or cartilage . Diarthrosis (dia-, through) • Freely movable © 2018 Pearson Education, Inc. Module 8.1: Joint classification Structural classification of joints . Fibrous • Suture (sutura, a sewing together) – Synarthrotic joint connected by dense fibrous connective tissue – Located between bones of the skull • Gomphosis (gomphos, bolt) – Synarthrotic joint binding teeth to bony sockets in maxillae and mandible © 2018 Pearson Education, Inc. -
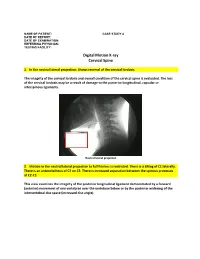
Digital Motion X-Ray Cervical Spine
NAME OF PATIENT: CASE STUDY 4 DATE OF REPORT: DATE OF EXAMINATION: REFERRING PHYSICIAN: TESTING FACILITY: Digital Motion X-ray Cervical Spine 1. In the neutral lateral projection: Shows reversal of the cervical lordosis. The integrity of the cervical lordosis and overall condition of the cervical spine is evaluated. The loss of the cervical lordosis may be a result of damage to the posterior longitudinal, capsular or interspinous ligaments. Neutral lateral projection 2. Motion in the neutral lateral projection to full flexion: Is restricted. There is a tilting of C1 laterally. There is an anterolisthesis of C2 on C3. There is increased separation between the spinous processes at C2-C3. This view examines the integrity of the posterior longitudinal ligament demonstrated by a forward (anterior) movement of one vertebrae over the vertebrae below or by the posterior widening of the intervertebral disc space (increased disc angle). Widening of posterior disc space Anterolisthesis The integrity of the interspinous ligament is evaluated in the lateral flexion view. Damage to this ligament results in increased separation of the spinous processes in flexion. Damaged Interspinous Ligament Full flexion projection 3. Motion in the neutral lateral projection to full extension: Is restricted. There is a retrolisthesis of C4 on C5. This view examines the integrity of the anterior longitudinal ligament demonstrated by a backward (posterior) movement of one vertebrae over the vertebrae below or by the anterior widening of the intervertebral disc space (increased disc angle). Retrolisthesis Widening of the anterior disc Full Extension 4. Motion in the oblique flexion projection: Is restricted. There is gapping of the facet joints at C6-C7 bilaterally and C7-T1 bilaterally. -

Epithelia Joitns
NAME LOCATION STRUCTURE FUNCTION MOVEMENT Temporomandibular joint Condylar head of ramus of Synovial Diarthrosis Modified hinge joint mandible and glenoid fossa of Rotation and gliding temporal bone Biaxial Zygapophyseal joint Between articular processes of Synovial Diarthrosis Gliding 2 adjacent vertebrae Non axial Atlanto-Occipital joints Atlas and occipital condyle of Synovial Diarthrosis Ellipsoid occipital bone Biaxial Atlantoaxial joints Atlas and axis Synovial Diarthrosis Pivot Uniaxial Joints of vertebral arches Ligaments Fibrous Amphiarthrosis Syndesmoses Intervertebral symphyseal Intervertebral disk between 2 Cartilaginous Amphiarthrosis joints vertebrae Symphysis Costovertebral Head of ribs and body of Synovial Diarthrosis Gliding thoracic vertebra Non axial Costotrasnverse joints Tubercle of rib and transverse Synovial Diarthrosis Gliding process of thoracic vertebra Non axial Lumbosacral Joint Left and right zygopophyseal Laterally Synovial joint Intervertebral symphyseal joint Symphysis SternoclavicularJoint Clavicular notch articulates Synovial Diarthrosis Gliding with medial ends of clavicle Non Axial Manubriosternal Joint Hyaline cartilage junction Cartilaginous Synarthrosis Sternal Angle between manubrium and body Symphysis Xiphisternal Joint Cartilage between xiphoid Synchondrosis Synarthrosis process and body Synostoses Sternocostal Joint (1st) Costocartilage 1 with sternum Cartilaginous Synchondrosis Synarthrosis NAME Location Section Anterior longitudinal runs down anterior surface of vertebral body Vertebral column ligament Posterior longitudinal in canal, runs down posterior surface of vertebral body ligament Interspinous ligament Connects spinous processes Ligamentum flavum Connects laminae ! Intra-articular Disc Between articulating surface of sternum and clavicle Sternoclavicular Joint Costoclavicular ligament 1st rib to clavicle !. -

Posterior Longitudinal Ligament Status in Cervical Spine Bilateral Facet Dislocations
Thomas Jefferson University Jefferson Digital Commons Department of Orthopaedic Surgery Faculty Papers Department of Orthopaedic Surgery November 2005 Posterior longitudinal ligament status in cervical spine bilateral facet dislocations John A. Carrino Harvard Medical School & Brigham and Women's Hospital Geoffrey L. Manton Thomas Jefferson University Hospital William B. Morrison Thomas Jefferson University Hospital Alex R. Vaccaro Thomas Jefferson University Hospital and The Rothman Institute Mark E. Schweitzer New York University & Hospital for Joint Diseases Follow this and additional works at: https://jdc.jefferson.edu/orthofp Part of the Orthopedics Commons LetSee next us page know for additional how authors access to this document benefits ouy Recommended Citation Carrino, John A.; Manton, Geoffrey L.; Morrison, William B.; Vaccaro, Alex R.; Schweitzer, Mark E.; and Flanders, Adam E., "Posterior longitudinal ligament status in cervical spine bilateral facet dislocations" (2005). Department of Orthopaedic Surgery Faculty Papers. Paper 3. https://jdc.jefferson.edu/orthofp/3 This Article is brought to you for free and open access by the Jefferson Digital Commons. The Jefferson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The Commons is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The Jefferson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in Department of Orthopaedic Surgery Faculty Papers by an authorized administrator of the Jefferson Digital Commons. For more information, please contact: [email protected]. -

Diagnostic Utility of Increased STIR Signal in the Posterior Atlanto-Occipital and Atlantoaxial Membrane Complex on MRI in Acute C1–C2 Fracture
Published July 6, 2017 as 10.3174/ajnr.A5284 ORIGINAL RESEARCH SPINE Diagnostic Utility of Increased STIR Signal in the Posterior Atlanto-Occipital and Atlantoaxial Membrane Complex on MRI in Acute C1–C2 Fracture X Y.-M. Chang, X G. Kim, X N. Peri, X E. Papavassiliou, X R. Rojas, and X R.A. Bhadelia ABSTRACT BACKGROUND AND PURPOSE: Acute C1–C2 fractures are difficult to detect on MR imaging due to a paucity of associated bone marrow edema. The purpose of this study was to determine the diagnostic utility of increased STIR signal in the posterior atlanto-occipital and atlantoaxial membrane complex (PAOAAM) in the detection of acute C1–C2 fractures on MR imaging. MATERIALS AND METHODS: Eighty-seven patients with C1–C2 fractures, 87 with no fractures, and 87 with other cervical fractures with acute injury who had both CT and MR imaging within 24 hours were included. All MR images were reviewed by 2 neuroradiologists for the presence of increased STIR signal in the PAOAAM and interspinous ligaments at other cervical levels. Sensitivity and specificity of increased signal within the PAOAAM for the presence of a C1–C2 fracture were assessed. RESULTS: Increased PAOAAM STIR signal was seen in 81/87 patients with C1–C2 fractures, 6/87 patients with no fractures, and 51/87 patients with other cervical fractures with 93.1% sensitivity versus those with no fractures, other cervical fractures, and all controls. Specificity was 93.1% versus those with no fractures, 41.4% versus those with other cervical fractures, and 67.2% versus all controls for the detection of acute C1–C2 fractures. -

Supraspinous Ligament Sprains
Unraveling the Mystery of Low Back Pain #5: Supraspinous Ligament Sprains Instructor: Ben Benjamin, Ph.D. Instructor: Ben Benjamin, Ph.D. [email protected] 1 SPONSOREDSPONSORED BY:BY: Over 30 years of experience building the finest portable treatment tables and accessories. Products that are visually stimulating, ergonomically supportive, and incredibly comfortable. The superior design and engineering capabilities merge to create the ultimate experience for you and your clients. www.oakworks.com 717.235.6807 SPONSOREDSPONSORED BY:BY: Mattes chair Side-lying position system Webinar Goal Explore the assessment and treatment of supraspinous ligament injuries: • Supraspinous ligaments L1-L5 • Suprasacral ligaments 2 Pretest 1. The supraspinous ligament is also known as the supraspinal ligament. True or False? 2. The suprasacral ligament connects the sacrum to the ilium. True or False? 3. The interspinous ligament is not continuous from vertebra to vertebra; it only connects two spinous processes to each other. True or False? 4. The supraspinous ligament in the low back limits lumbar flexion. True or False? 5. The posterior layer of thoracolumbar fascia and multifidus muscles combine to form the lumbar supraspinous ligaments. True or False? 6. The suprasacral ligament holds the sacrum to the pelvis. True or False? Anatomy Anatomy of the Supraspinous Ligaments • Connect all five lumbar vertebrae • Connect L5 to the sacrum • Sometimes called the supraspinal ligaments 3 Anatomy of the Supraspinous Ligaments • Run between the tips of the spinous -

Development, Validation and Clinical Application of Finite Element Human Pelvis Model
Health Science Campus FINAL APPROVAL OF THESIS Master of Science in Biomedical Sciences Development, validation and clinical application of finite element human pelvis model Submitted by: Alexander A. Ivanov In partial fulfillment of the requirements for the degree of Master of Science in Biomedical Sciences Examination Committee Signature/Date Major Advisor: Nabil Ebraheim, M.D. Academic Vijay Goel, Ph.D. Advisory Committee: Ashok Biyani, M.D. Senior Associate Dean College of Graduate Studies Michael S. Bisesi, Ph.D. Date of Defense: May 15, 2008 A Thesis Entitled Development, Validation and Clinical Application of the Finite Element Model of Human Pelvis. By Alexander A. Ivanov, M.D. Submitted as partial fulfillment of the requirements for the Master of Science in Orthopaedic Science ______________________________ Advisor: Dr. Nabil A. Ebraheim, M.D. ______________________________ Co-Advisor: Dr. Vijay K. Goel, Ph.D. ______________________________ Graduate School The University of Toledo 2008 1 © 2008, Alexander A. Ivanov 2 College of Health Science I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY: Alexander A Ivanov, M.D. ENTITLED: Development, validation and clinical application of finite element human pelvis model BE ACCEPTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF: Master of Science in Orthopaedic Science ______________________________________________________________ Thesis Advisor: Dr. Nabil A. Ebraheim, M.D. _______________________________________________________________ Thesis Co-Advisor: Dr. Vijay K. Goel, Ph.D. Recommendation concurred by: ___________________________________ Committee Dr.Ashok Biyani, M.D. Of Final Examination ________________________________________________________________ Dean, College of Health Science 3 Acknowledgment I would like to extend my gratitude to my advisors Dr. Nabil Ebraheim and Dr. Vijay Goel for their incredible support of this work. -

Degenerative Changes in the Interspinous Ligament
ORIGINAL ARTICLE Acta Orthop Traumatol Turc 2014;48(6):661-666 doi: 10.3944/AOTT.2014.13.0149 Degenerative changes in the interspinous ligament Jian-Feng ZHANG*, Chao LIU*, He-Jun YU, Jian-Jun MA, Hong-Xin CAI, Shun-Wu FAN Department of Orthopedics, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China Objective: The aim of this study was to investigate the imaging assessment of interspinous ligament degeneration (ISLD) in patients with or without low back pain (LBP). Methods: Sixty patients with LBP were enrolled in Group A and 60 subjects frequency-matched by age and sex in Group B. An MRI-based grading system for ISLD was scored and ranged from Type A (nor- mal) to Type D (severe). The lumbar disc was also graded according to degeneration at four lumbar levels. Results: Type A ISLD was the most prevalent type with 161 levels (67.1%) in Group A and 172 (71.7%) in Group B. Type D was the least frequent, seen in 13 levels in Group A and 3 in Group B. There was a significantly higher incidence of Type D ISLD in Group A than Group B (5.4% vs. 1.3%, p<0.05). The average age of patients with Type D ISLD in Group A was higher than Types A, B and C (Type A and B p<0.01, Type C p<0.05). In Group B, the age of patients with Type D ISLD was sig- nificantly higher than those with Type A (p<0.05). Although disc grade increased in advanced ISLD in both groups, only the difference between Type D and Types A and B in Group A were statistically significant (p<0.05). -

Perry Dhaliwal, Pgy-1 University of Calgary Overview
lumbosacral spine: anatomy, radiology and biomechanics perry dhaliwal, pgy-1 university of calgary overview ‣osteology ‣muscles of the lower back ‣ligaments of the lumbar spine ‣vascular supply ‣nerves and lumbosacral plexus ‣radiology correlates ‣biomechanics of the lumbosacral spine vertebral column osteology: lumbar spine x-ray of lumbar spine axial ct intervertebral disc and facet joints intervertebral disc and facet joints osteology: sacrum osteology: sacrum muscles of the lower back muscles of the back superficial layer muscles of the back intermediate layer deep layer ligaments of the lumbar spine lumbar ligaments •anterior longitudinal ligament •posterior longitudinal ligament •ligamentum flavum •interspinous ligament •supraspinous ligament sacral ligaments •iliolumbar ligament •posterior sacroiliac ligaments •sacrospinous ligament •sacrotuberous ligament t2 sagittal t2 axial vascular supply arterial supply venous drainage nerves and lumbosacral plexus spinal nerve roots spinal nerve roots spinal nerve roots l p ef p f d lumbosacral plexus biomechanics of the lumbar spine physical properties of the spine ‣intervertebral disc ‣subject to compressive, tensile and torsional loads ‣responsible for carrying all the compressive loading (along with facet joints) ‣biomechanics of the disc are dependent on its state of degeneration ‣most degenerated discs: l3-l4, l4-l5, l5-s1 ‣composed of nucleus pulposus, annulus fibrosus and cartilaginous end-plate physical properties of the spine ‣spinal ligaments ‣most effective in carrying loads in -

Primary Cervical Spine Ligaments
Strain Rate Dependent Properties of Younger Human Cervical Spine Ligaments by Stephen Mattucci A thesis presented to the University of Waterloo in fulfillment of the thesis requirement for the degree of Master of Applied Science in Mechanical Engineering Waterloo, Ontario, Canada, 2011 ©Stephen Mattucci 2011 AUTHOR'S DECLARATION I hereby declare that I am the sole author of this thesis. This is a true copy of the thesis, including any required final revisions, as accepted by my examiners. I understand that my thesis may be made electronically available to the public. Stephen Frank Ernesto Mattucci ii Abstract The cervical spine ligaments play an essential role in limiting the physiological ranges of motion in the neck; however, traumatic loading such as that experienced in automotive crash scenarios can lead to ligament damage and result in neck injury. The development of detailed finite element models for injury simulation requires accurate ligament mechanical properties at relevant loading rates. The objective of this research was to provide detailed mechanical properties for the cervical spine ligaments, by performing tensile tests at elongation rates relevant to automobile crash scenarios, using younger specimens (less than 50 years old), and to provide a comprehensive investigation of spinal level and gender effects. The five primary ligaments (present between C2-T1) investigated were: the anterior longitudinal ligament, posterior longitudinal ligament, capsular ligament, ligamentum flavum, and interspinous ligament. The craniovertebral ligaments (Skull/C0-C2) investigated were the tectorial membrane/vertical cruciate/apical/alar ligament complex, transverse ligament, anterior atlanto- occipital membrane, posterior atlanto-occipital membrane, anterior atlanto-axial membrane, and posterior atlanto-axial membrane. -

Mechanical Role of the Spine, Ribcage and Interabdominal Pressure in The
DEVELOPMENT OF A NON-FUSION SCOLIOSIS CORRECTION DEVICE NUMERICAL MODELLING OF SCOLIOSIS CORRECTION This project, “A non-fusion scoliosis correction device”, was supported by a grant from the Dutch Technology Foundation (STW), applied science division of NWO and the Technology Program of the ministry of economic affairs (project number 07618). The printing of this thesis was financially supported by: Stichting Technologische Wetenschappen (STW) Samenstelling promotiecommissie: Voorzittert en secretaris: Prof. dr. F. Eising Universiteit Twente Promotoren: Prof. dr. ir. G.J. Verkerke Universiteit Twente Prof. dr. A.G. Veldhuizen Universitair Medisch Centrum Groningen Assistent promotor: Dr. ir. J.J. Homminga Universiteit Twente Leden: Prof. dr. ir. N.J.J. Verdonschot Universiteit Twente Prof. dr. ir. A. De Boer Universiteit Twente Prof. dr. K. Ito Technische Universiteit Eindhoven Prof. dr. J.H. van Dieën Vrije Universiteit Amsterdam Prof. dr. ir. N.M. Maurits Universitair Medisch Centrum Groningen Paranimfen: Tjitske Boonstra Evelien Platvoet Printed by: Ipskamp Drukkers BV, Enschede ISBN: 978-90-365-3229-7 Copyright © 2011 by G.J.M. Meijer, Enschede, The Netherlands. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording or any information storage or retrieval system, without permission in writing from the author. DEVELOPMENT OF A NON-FUSION SCOLIOSIS CORRECTION DEVICE NUMERICAL MODELLING OF SCOLIOSIS CORRECTION PROEFSCHRIFT ter verkrijging van de graad van doctor aan de Universiteit Twente, op gezag van de rector magnificus, prof. dr. H. Brinksma, volgens besluit van het College voor Promoties in het openbaar te verdedigen op vrijdag 14 oktober 2011 om 12.45 uur door Gerarda Johanna Maria Meijer geboren op 6 december 1978 te Oldenzaal Dit proefschrift is goedgekeurd door: Prof. -

Ossification of Interspinous and Supraspinous Ligaments of the Adult 5Th Lumbar Vertebra and Its Clinical Significance- a Case Report
IOSR Journal of Dental and Medical Sciences (JDMS) ISSN: 2279-0853, ISBN: 2279-0861. Volume 1, Issue 5 (Sep-Oct. 2012), PP 27-28 www.iosrjournals.org Ossification of Interspinous and Supraspinous Ligaments of the Adult 5th Lumbar Vertebra and Its Clinical Significance- A Case Report. 1Dr. S. Saritha, 2M. Preevan Kumar, 3Dr. G. Supriya 1(professor of anatomy), 2(lecture), (3asst.professor of anatomy) Abstract: Human Axial Skeleton has drawn much interest for Medical researchers because of the Upright posture. The human vertebral column plays an important role in stability and weight transmission. It is adapted to protect the spinal cord. Congenital or acquired anomalies are common in the vertebral column. At the same time the vertebral column is the site for many orthopedic disorders which may be pathological or developmental, leading to instability, low back pain, kyphosis, scoliosis and Ankylosing spondylitis. During routine osteology classes, processed lumbar vertebrae were collected to explain to the students of I M.B.B.S. The author realized abnormal 5th lumbar vertebra. It had ossification of Interspinous and Supraspinous ligaments may be a feature of Ankylosis Spondylitis. This condition has a genetic and clinical importance. The purpose of present study is to highlight the I M.B.B.S. students about abnormal vertebrae and their clinical applications. This abnormal 5th lumbar vertebra was studied in detail with regards to its general morphology aspects, genetic factors, external stimulus and clinical manifestations. Key word: Supraspinous and Interspinous ligaments (SS&IS), ossification, calcification, 5th lumbar vertebra, Ankylosing spondylitis (AS). I. Introduction The vertebral column preforms the important function of weight bearing and transmission.