Development, Validation and Clinical Application of Finite Element Human Pelvis Model
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synovial Joints Permit Movements of the Skeleton
8 Joints Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Joint Structure and Movement Learning Outcomes 8.1 Contrast the major categories of joints, and explain the relationship between structure and function for each category. 8.2 Describe the basic structure of a synovial joint, and describe common accessory structures and their functions. 8.3 Describe how the anatomical and functional properties of synovial joints permit movements of the skeleton. © 2018 Pearson Education, Inc. Section 1: Joint Structure and Movement Learning Outcomes (continued) 8.4 Describe flexion/extension, abduction/ adduction, and circumduction movements of the skeleton. 8.5 Describe rotational and special movements of the skeleton. © 2018 Pearson Education, Inc. Module 8.1: Joints are classified according to structure and movement Joints, or articulations . Locations where two or more bones meet . Only points at which movements of bones can occur • Joints allow mobility while preserving bone strength • Amount of movement allowed is determined by anatomical structure . Categorized • Functionally by amount of motion allowed, or range of motion (ROM) • Structurally by anatomical organization © 2018 Pearson Education, Inc. Module 8.1: Joint classification Functional classification of joints . Synarthrosis (syn-, together + arthrosis, joint) • No movement allowed • Extremely strong . Amphiarthrosis (amphi-, on both sides) • Little movement allowed (more than synarthrosis) • Much stronger than diarthrosis • Articulating bones connected by collagen fibers or cartilage . Diarthrosis (dia-, through) • Freely movable © 2018 Pearson Education, Inc. Module 8.1: Joint classification Structural classification of joints . Fibrous • Suture (sutura, a sewing together) – Synarthrotic joint connected by dense fibrous connective tissue – Located between bones of the skull • Gomphosis (gomphos, bolt) – Synarthrotic joint binding teeth to bony sockets in maxillae and mandible © 2018 Pearson Education, Inc. -

Peripartum Pubic Symphysis Diastasis—Practical Guidelines
Journal of Clinical Medicine Review Peripartum Pubic Symphysis Diastasis—Practical Guidelines Artur Stolarczyk , Piotr St˛epi´nski* , Łukasz Sasinowski, Tomasz Czarnocki, Michał D˛ebi´nski and Bartosz Maci ˛ag Department of Orthopedics and Rehabilitation, Medical University of Warsaw, 02-091 Warsaw, Poland; [email protected] (A.S.); [email protected] (Ł.S.); [email protected] (T.C.); [email protected] (M.D.); [email protected] (B.M.) * Correspondence: [email protected] Abstract: Optimal development of a fetus is made possible due to a lot of adaptive changes in the woman’s body. Some of the most important modifications occur in the musculoskeletal system. At the time of childbirth, natural widening of the pubic symphysis and the sacroiliac joints occur. Those changes are often reversible after childbirth. Peripartum pubic symphysis separation is a relatively rare disease and there is no homogeneous approach to treatment. The paper presents the current standards of diagnosis and treatment of pubic diastasis based on orthopedic and gynecological indications. Keywords: pubic symphysis separation; pubic symphysis diastasis; pubic symphysis; pregnancy; PSD 1. Introduction The proper development of a fetus is made possible due to numerous adaptive Citation: Stolarczyk, A.; St˛epi´nski,P.; changes in women’s bodies, including such complicated systems as: endocrine, nervous Sasinowski, Ł.; Czarnocki, T.; and musculoskeletal. With regard to the latter, those changes can be observed particularly D˛ebi´nski,M.; Maci ˛ag,B. Peripartum Pubic Symphysis Diastasis—Practical in osteoarticular and musculo-ligamento-fascial structures. Almost all of those changes Guidelines. J. Clin. Med. -
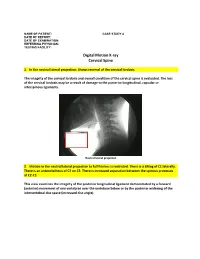
Digital Motion X-Ray Cervical Spine
NAME OF PATIENT: CASE STUDY 4 DATE OF REPORT: DATE OF EXAMINATION: REFERRING PHYSICIAN: TESTING FACILITY: Digital Motion X-ray Cervical Spine 1. In the neutral lateral projection: Shows reversal of the cervical lordosis. The integrity of the cervical lordosis and overall condition of the cervical spine is evaluated. The loss of the cervical lordosis may be a result of damage to the posterior longitudinal, capsular or interspinous ligaments. Neutral lateral projection 2. Motion in the neutral lateral projection to full flexion: Is restricted. There is a tilting of C1 laterally. There is an anterolisthesis of C2 on C3. There is increased separation between the spinous processes at C2-C3. This view examines the integrity of the posterior longitudinal ligament demonstrated by a forward (anterior) movement of one vertebrae over the vertebrae below or by the posterior widening of the intervertebral disc space (increased disc angle). Widening of posterior disc space Anterolisthesis The integrity of the interspinous ligament is evaluated in the lateral flexion view. Damage to this ligament results in increased separation of the spinous processes in flexion. Damaged Interspinous Ligament Full flexion projection 3. Motion in the neutral lateral projection to full extension: Is restricted. There is a retrolisthesis of C4 on C5. This view examines the integrity of the anterior longitudinal ligament demonstrated by a backward (posterior) movement of one vertebrae over the vertebrae below or by the anterior widening of the intervertebral disc space (increased disc angle). Retrolisthesis Widening of the anterior disc Full Extension 4. Motion in the oblique flexion projection: Is restricted. There is gapping of the facet joints at C6-C7 bilaterally and C7-T1 bilaterally. -

Lower Back Pain and the Sacroiliac Joint What Is the Sacroiliac Joint?
PATIENT INFORMATION Lower Back Pain and the Sacroiliac Joint What is the Sacroiliac Joint? Your Sacroiliac (SI) joint is formed by the connection of the sacrum and iliac bones. These two large bones are part of the pelvis Sacroiliac and are held together by a collection of joint ligaments. The SI joint supports the weight of your upper body which places a large amount of stress across your SI joint. What is Sacroiliac Joint Disorder? The SI joint is a documented source of lower back pain. The joint is the most likely source of pain in 30% of patients with lower back pain. Pain caused by sacroiliac joint disorder can be felt in the lower back, buttocks, or legs. Sacroiliac joint fixation is indicated in patients with severe, chronic sacroiliac joint pain who have failed extensive conservative measures, or in acute cases of trauma. What are potential symptoms? • Lower back pain • Lower extremity pain (numbness, tingling, weakness) • Pelvis/buttock pain • Hip/groin pain • Unilateral leg instability (buckling, giving away) • Disturbed sleep patterns • Disturbed sitting patterns (unable to sit for long periods of time on one side) • Pain going away from sitting to standing How is Sacroiliac Joint Disorder diagnosed? Sacroiliac joint disorder is diagnosed by the patient’s history, physical findings, radiological investigations and SI joint injections. Sacroiliac injection, which is the gold standard for confirming SI joint disorder will be delivered with fluoroscopic or CT guidance to validate accurate placement of the needle in the SI joint. What is the Orthofix SambaScrew®? Your surgeon has chosen the SambaScrew because it utilizes a minimally invasive surgical technique to sacroiliac fixation. -

Epithelia Joitns
NAME LOCATION STRUCTURE FUNCTION MOVEMENT Temporomandibular joint Condylar head of ramus of Synovial Diarthrosis Modified hinge joint mandible and glenoid fossa of Rotation and gliding temporal bone Biaxial Zygapophyseal joint Between articular processes of Synovial Diarthrosis Gliding 2 adjacent vertebrae Non axial Atlanto-Occipital joints Atlas and occipital condyle of Synovial Diarthrosis Ellipsoid occipital bone Biaxial Atlantoaxial joints Atlas and axis Synovial Diarthrosis Pivot Uniaxial Joints of vertebral arches Ligaments Fibrous Amphiarthrosis Syndesmoses Intervertebral symphyseal Intervertebral disk between 2 Cartilaginous Amphiarthrosis joints vertebrae Symphysis Costovertebral Head of ribs and body of Synovial Diarthrosis Gliding thoracic vertebra Non axial Costotrasnverse joints Tubercle of rib and transverse Synovial Diarthrosis Gliding process of thoracic vertebra Non axial Lumbosacral Joint Left and right zygopophyseal Laterally Synovial joint Intervertebral symphyseal joint Symphysis SternoclavicularJoint Clavicular notch articulates Synovial Diarthrosis Gliding with medial ends of clavicle Non Axial Manubriosternal Joint Hyaline cartilage junction Cartilaginous Synarthrosis Sternal Angle between manubrium and body Symphysis Xiphisternal Joint Cartilage between xiphoid Synchondrosis Synarthrosis process and body Synostoses Sternocostal Joint (1st) Costocartilage 1 with sternum Cartilaginous Synchondrosis Synarthrosis NAME Location Section Anterior longitudinal runs down anterior surface of vertebral body Vertebral column ligament Posterior longitudinal in canal, runs down posterior surface of vertebral body ligament Interspinous ligament Connects spinous processes Ligamentum flavum Connects laminae ! Intra-articular Disc Between articulating surface of sternum and clavicle Sternoclavicular Joint Costoclavicular ligament 1st rib to clavicle !. -

Posterior Longitudinal Ligament Status in Cervical Spine Bilateral Facet Dislocations
Thomas Jefferson University Jefferson Digital Commons Department of Orthopaedic Surgery Faculty Papers Department of Orthopaedic Surgery November 2005 Posterior longitudinal ligament status in cervical spine bilateral facet dislocations John A. Carrino Harvard Medical School & Brigham and Women's Hospital Geoffrey L. Manton Thomas Jefferson University Hospital William B. Morrison Thomas Jefferson University Hospital Alex R. Vaccaro Thomas Jefferson University Hospital and The Rothman Institute Mark E. Schweitzer New York University & Hospital for Joint Diseases Follow this and additional works at: https://jdc.jefferson.edu/orthofp Part of the Orthopedics Commons LetSee next us page know for additional how authors access to this document benefits ouy Recommended Citation Carrino, John A.; Manton, Geoffrey L.; Morrison, William B.; Vaccaro, Alex R.; Schweitzer, Mark E.; and Flanders, Adam E., "Posterior longitudinal ligament status in cervical spine bilateral facet dislocations" (2005). Department of Orthopaedic Surgery Faculty Papers. Paper 3. https://jdc.jefferson.edu/orthofp/3 This Article is brought to you for free and open access by the Jefferson Digital Commons. The Jefferson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The Commons is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The Jefferson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in Department of Orthopaedic Surgery Faculty Papers by an authorized administrator of the Jefferson Digital Commons. For more information, please contact: [email protected]. -

Chapter 02: Netter's Clinical Anatomy, 2Nd Edition
Hansen: Netter's Clinical Anatomy, 2nd Edition - with Online Access 2 BACK 1. INTRODUCTION 4. MUSCLES OF THE BACK REVIEW QUESTIONS 2. SURFACE ANATOMY 5. SPINAL CORD 3. VERTEBRAL COLUMN 6. EMBRYOLOGY FINAL 1. INTRODUCTION ELSEVIERl VertebraeNOT prominens: the spinous process of the C7- vertebra, usually the most prominent The back forms the axis (central line) of the human process in the midline at the posterior base of body and consists of the vertebral column, spinal cord, the neck supporting muscles, and associated tissues (skin, OFcon- l Scapula: part of the pectoral girdle that sup- nective tissues, vasculature, and nerves). A hallmark of ports the upper limb; note its spine, inferior human anatomy is the concept of “segmentation,” and angle, and medial border the back is a prime example. Segmentation and bilat l Iliac crests: felt best when you place your eral symmetry of the back will be obvious as you hands “on your hips”; an imaginary horizontal study the vertebral column, the distribution of the line connecting the crests passes through the spinal nerves, the muscles of th back, and its vascular spinous process of the L4 vertebra and the supply. intervertebral disc of L4-L5, a useful landmark Functionally, the back is involved in three primary for a lumbar puncture or epidural block tasks: l Posterior superior iliac spines: an imaginary CONTENThorizontal line connecting these two points l Support: the vertebral column forms the axis of passes through the spinous process of S2 (second the body and is critical for our upright posture sacral segment) (standing or si ting), as a support for our head, as an PROPERTYattachment point and brace for move- 3. -

Chronic Sacroiliac Joint and Pelvic Girdle Pain and Dysfunction
Chronic Sacroiliac Joint and Pelvic Girdle Pain and Dysfunction Successfully Holly Jonely, PT, ScD, FAAOMPT1,3 Melinda Avery, PT, DPT1 Managed with a Multimodal and Mehul J. Desai, MD, MPH2,3 Multidisciplinary Approach: A Case Series 1The George Washington University, Department of Health, Human Function and Rehabilitation Sciences, Program in Physical Therapy, Washington, DC 2The George Washington University, School of Medicine & Health Sciences, Department of Anesthesia & Critical Care, Washington, DC 3International Spine, Pain & Performance Center, Washington, DC ABSTRACT PGP, impairments of the SIJ are not lim- Case 2 Background and Purpose: Sacroiliac ited to intraarticular pain and often include A 30-year-old nulliparous female with joint (SIJ) or pelvic girdle pain (PGP) account impairments of the surrounding muscles or a chronic history of right posterior pelvic for 20-40% of all low back pain cases in the connective tissues, as well as, aberrant and pain following an injury as a college athlete United States. Diagnosis and management asymmetrical movement patterns within the participating in crew. She reported slipping of these disorders can be challenging due to region of the lumbo-pelvic-hip complex.7 in a boat and falling onto her sacrum. Her limited and conflicting evidence in the lit- These impairments have a negative impact previous conservative management included erature and the varying patient presentation. on the PG’s role in support and load trans- physical therapy that emphasized pelvic The purpose of this case series is to describe fer between the lower extremities and trunk. manipulations, use of a pelvic belt, and stabi- the outcome observed in 3 patients present- This ariabilityv in observed impairments lization exercises. -

The Sacroiliac Problem: Review of Anatomy, Mechanics, and Diagnosis
The sacroiliac problem: Review of anatomy, mechanics, and diagnosis MYRON C. BEAL, DD., FAAO East Lansing, Michigan methods have evolved along with modifications in Studies of the anatomy of the the hypotheses. Unfortunately, definitive analysis sacroiliac joint are reviewed, of the sacroiliac joint problem has yet to be including joint changes associated achieved. with aging and sex. Both descriptive Two excellent reviews of the medical literature and analytical investigations of joint on the sacroiliac joint are by Solonen i and a three- movement are presented, as well as part series by Weisl. clinical hypotheses of sacroiliac joint The present treatise will review the anatomy of motion. The diagnosis of sacroiliac the sacroiliac joint, studies of sacroiliac move- joint dysfunction is described in ment, hypotheses of sacroiliac mechanics, and the detail. diagnosis of sacroiliac dysfunction. Anatomy The formation of the sacroiliac joint begins during the tenth week of intrauterine life, and the joint is fully developed by the seventh month. The joint In recent years it has been generally recognized surfaces remain flat until sometime after puberty; that the sacroiliac joints are capable of movement. smooth surfaces in the adult are the exception. The clinical significance of sacroiliac motion, or The contour of the joint surface continues to lack of motion, is still subject to debate. The role of change with age. 2m In the third and fourth decades the sacroiliac joints in body mechanics can be illus- there is an increase in the number and size of the trated by a mechanical analogy. A 1 to 2 mm. mal- elevations and depressions, which interlock and alignment of a bearing in a machine can cause ab- limit mobility. -

Sacroiliac Joint Dysfunction a Case Study
NOR200188.qxd 3/8/11 9:53 PM Page 126 Sacroiliac Joint Dysfunction A Case Study CPT William Murray Pain is a widespread issue in the United States. Nine of physical therapist. She was evaluated and her treatment 10 Americans regularly suffer from pain, and nearly every consisted of a transcutaneous electrical nerve stimula- person will experience low back pain at one point in their lives. tion unit while in the PT clinic, aqua therapy, and ice Undertreated or unrelieved pain costs more than and heat application. $60 billion a year from decreased productivity, lost income, After several weeks, Ms. T returned to the primary care and medical expenses. The ability to diagnose and provide ap- provider and informed her that the pain has not decreased and “feels like that it is getting worse.” She also informed propriate medical treatment is imperative. This case study ex- the provider that she was having difficulty sleeping and amines a 23-year-old Active Duty woman who is preparing to constantly feeling tired secondary to pain. Throughout the be involuntarily released from military duty for an easily cor- next several months, the primary care provider tried nu- rectable medical condition. She has complained of chronic low merous medication trials with no relief for the patient. Ms. back pain that radiates into her hip and down her leg since ex- T gives a history of being prescribed numerous medica- periencing a work-related injury. She has been seen by numer- tions within several drug classifications. She stated vari- ous providers for the previous 11 months before being referred ous side effects that are related to the medications and to the chronic pain clinic. -

Successful Treatment of Supraspinous and Interspinous Ligament Injury with Ultrasound-Guided Platelet-Rich Plasma Injection
HSSXXX10.1177/1556331621992312HSS Journal®: The Musculoskeletal Journal of Hospital for Special SurgeryCreighton et al 992312case-report2021 Case Report HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery Successful Treatment of Supraspinous 1 –4 © The Author(s) 2021 Article reuse guidelines: and Interspinous Ligament Injury sagepub.com/journals-permissions DOI:https://doi.org/10.1177/1556331621992312 10.1177/1556331621992312 With Ultrasound-Guided Platelet-Rich journals.sagepub.com/home/hss Plasma Injection: Case Series Andrew Creighton, DO1, Roger A. Sanguino, MS1, Jennifer Cheng, PhD1, and James F. Wyss, MD, PT1 Keywords supraspinous ligament, interspinous ligament, platelet-rich plasma, ultrasound, nonoperative treatments, lumbar spine Received October 18, 2020. Accepted October 21, 2020. Introduction running volume. The LBP intensity ranged from 3 to 9/10 and was worse with prolonged standing, sitting, or running. Low back pain (LBP) is a very common complaint and is He reported no improvement with 3 prior courses of PT, now the number one cause of disability across the globe NSAIDs, and use of a seat cushion. Physical examination [5,13]. Both the supraspinous ligament (SSL) and interspi- revealed mild right thoracolumbar curvature. Tenderness nous ligament (ISL) form part of the posterior ligamentous was appreciated over the L5 spinous process and interspi- complex, which is believed to play an integral role in the nous region above and below L5. Strength, sensation, and stability of the thoracolumbar spine [8]. The SSL begins at reflexes were normal. the C7 spinous process and extends to L3 and L4 in 22% Radiographs were unremarkable. Magnetic resonance and 74% of adults, respectively [11]. -

Diagnostic Utility of Increased STIR Signal in the Posterior Atlanto-Occipital and Atlantoaxial Membrane Complex on MRI in Acute C1–C2 Fracture
Published July 6, 2017 as 10.3174/ajnr.A5284 ORIGINAL RESEARCH SPINE Diagnostic Utility of Increased STIR Signal in the Posterior Atlanto-Occipital and Atlantoaxial Membrane Complex on MRI in Acute C1–C2 Fracture X Y.-M. Chang, X G. Kim, X N. Peri, X E. Papavassiliou, X R. Rojas, and X R.A. Bhadelia ABSTRACT BACKGROUND AND PURPOSE: Acute C1–C2 fractures are difficult to detect on MR imaging due to a paucity of associated bone marrow edema. The purpose of this study was to determine the diagnostic utility of increased STIR signal in the posterior atlanto-occipital and atlantoaxial membrane complex (PAOAAM) in the detection of acute C1–C2 fractures on MR imaging. MATERIALS AND METHODS: Eighty-seven patients with C1–C2 fractures, 87 with no fractures, and 87 with other cervical fractures with acute injury who had both CT and MR imaging within 24 hours were included. All MR images were reviewed by 2 neuroradiologists for the presence of increased STIR signal in the PAOAAM and interspinous ligaments at other cervical levels. Sensitivity and specificity of increased signal within the PAOAAM for the presence of a C1–C2 fracture were assessed. RESULTS: Increased PAOAAM STIR signal was seen in 81/87 patients with C1–C2 fractures, 6/87 patients with no fractures, and 51/87 patients with other cervical fractures with 93.1% sensitivity versus those with no fractures, other cervical fractures, and all controls. Specificity was 93.1% versus those with no fractures, 41.4% versus those with other cervical fractures, and 67.2% versus all controls for the detection of acute C1–C2 fractures.