A Double Peak in the Seasonality of California's
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Climate Change Impacts on Water Management in the Puget Sound Region, Washington, USA Julie A
Climate Change Impacts on Water Management in the Puget Sound Region, Washington, USA Julie A. Vano1, Nathalie Voisin1, Lan Cuo1,2, Alan F. Hamlet1,2, Marketa McGuire Elsner2, Richard N. Palmer3, Austin Polebitski1, and Dennis P. Lettenmaier1,2 Abstract limate change is projected to result, on average, in earlier snowmelt and reduced summer flows, patterns that are not well represented in the historical observations used for planning and reliability analyses by water utilities. CWe extend ongoing efforts in the Puget Sound basin cities of Everett, Seattle, and Tacoma to characterize differences between historic and future streamflow and the ability of the region’s water supply systems to meet future demands. We use future streamflow simulations for the 2020s, 2040s, and 2080s from the Distributed Hydrology-Soil- Vegetation Model (DHSVM), driven by climate simulations archived by the 2007 Fourth Assessment Report (AR4) of the Intergovernmental Panel on Climate Change (IPCC). We use ensembles of streamflow predictions produced by DHSVM forced with multiple downscaled ensembles from the IPCC climate models as inputs to reservoir system models for the Everett, Seattle, and Tacoma water supply systems. Over the next century, under average conditions all three systems are projected to experience a decline and eventual disappearance of the springtime snowmelt peak in their inflows. How these shifts impact water management depends on the specifics of the reservoir system and their operating objectives, site-specific variations in the influence that reductions in snowmelt have on reservoir inflows, and the adaptive capacity of each system. Without adaptations, average seasonal drawdown of reservoir storage is projected to increase in all of the systems throughout the 21st century. -

Analysis of Runoff from Small Drainage Basins in Wyoming
Analysis of Runoff from Small Drainage Basins in Wyoming GEOLOGICAL SURVEY WATER-SUPPLY PAPER 2056 Prepared In cooperation with the Wyoming Highway Department and the U.S. Department of Transportation, Federal Highway Administration Analysis of Runoff from Small Drainage Basins in Wyoming By GORDON S. CRAIG, JR., and JAMES G. RANKL GEOLOGICAL SURVEY WATER-SUPPLY PAPER 2056 Prepared in cooperation with the Wyoming Highway Department and the U.S. Department of Transportation, Federal Highway Administration UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1978 UNITED STATES DEPARTMENT OF THE INTERIOR CECIL D. ANDRUS, Secretary GEOLOGICAL SURVEY H. William Menard, Director Library of Congress Catalog-card Number 78-600090 For sale by the Superintendent of Documents, U. S. Government Printing OfEce Washington, D. C. 20402 Stock Number 024-001-03106-0 CONTENTS Page Symbols_________________________________________ V Abstract_________________________________________ 1 Introduction ________________________________________ 1 Purpose and scope _______ ____________________________ 1 Limitations of study _______________________________ 5 Acknowledgments ________________________________________ 5 Use of metric units of measurement ________________________ 6 Data collection ____________________________________ 6 Description of area __________________________________ 6 Instrumentation __________________________________ 7 Types of records _________________________________________ 7 Station frequency analysis _________________________________ 9 Runoff -

A Double Peak in the Seasonality of California's
Biogeosciences, 17, 405–422, 2020 https://doi.org/10.5194/bg-17-405-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. A double peak in the seasonality of California’s photosynthesis as observed from space Alexander J. Turner1,2,3, Philipp Köhler4, Troy S. Magney3,4,5, Christian Frankenberg3,4, Inez Fung1, and Ronald C. Cohen1,2 1Department of Earth and Planetary Sciences, University of California, Berkeley, CA 94720, USA 2College of Chemistry, University of California, Berkeley, CA 94720, USA 3Jet Propulsion Laboratory, California Institute of Technology, Pasadena, CA 91109, USA 4Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA 91226, USA 5Department of Plant Sciences, University of California, Davis, CA 95616, USA Correspondence: Alexander J. Turner ([email protected]) Received: 24 September 2019 – Discussion started: 26 September 2019 Revised: 16 December 2019 – Accepted: 6 January 2020 – Published: 29 January 2020 Abstract. Solar-induced chlorophyll fluorescence (SIF) has nia’s photosynthesis is due to two processes that are out of been shown to be a powerful proxy for photosynthesis and phase: grasses, chaparral, and oak savanna ecosystems show gross primary productivity (GPP). The recently launched an April maximum, while evergreen forests peak in June. TROPOspheric Monitoring Instrument (TROPOMI) features An empirical orthogonal function (EOF) analysis corrobo- the required spectral resolution and signal-to-noise ratio to rates the phase offset and spatial patterns driving the double retrieve SIF from space. Here, we present a downscaling peak. The EOF analysis further indicates that two spatiotem- method to obtain 500 m spatial resolution SIF over Cal- poral patterns explain 84 % of the variability in the SIF data. -
Moramap1.Pdf
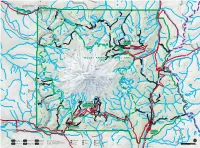
To Wilkeson 13mi / 21km from CLEARWATER Carbon River Entrance k Obtain Climbing and Wilderness Road closed to vehicles beyond e this point. Road open to foot WILDERNESS e Camping Permits for the northwest r and bicycle traffic. Bicyclists must C r area of the park at Carbon River remain on the main road. e v Carbon e i Ranger Station. MT. BAKER-SNOQUALMIE NATIONAL FOREST o D R 165 rbon River T Ca rail (former road) r r 4mi e e C v Carbon River Entrance 6km i G v Chenuis Falls E i h e 410 R o G Lake t 1880ft k R e Carbon River i a e 573m n D e t I Eleanor e h u t Carbon River Rainforest Trail r R i k Tirzah Peak i C s h W e J 5208ft Scarface u e Adelaide Pigeon Peak r W E k n C 1587m 6108ft e L o C Lake e E k 1862m re C s N Oliver r r C Wallace Peak C A t e E e C G Ranger Falls o Sweet H Lake d k r F E D D a Peak e I N N e U ls t R I E s k l S Marjorie e a C P F Slide Mountain r E W T Lake C M e Green D 6339ft 2749ft N46° 58´ 42˝ S r Ipsut Creek O e e N 1932m 838m U Lake k I U e W121° 32´ 07˝ Florence Peak N Chenuis y R r C T Cr r k 5508ft e a A r A rb B e L 1679m g o EE I N Lakes rn n n F o b K Arthur Peak LA e I a T Lake H l Gove Peak S 5483ft R n k i C NORTH C l 5310ft Ethel a c v R 1671m u J r e E PARK 1619m W R V o e r iv H s S o e T n e ep r k de Lake K h k rl R in BURNT e an James A e C Howard Peak e d Y P PARK r r E Tyee Peak C LL e 5683ft Tr OW Natural D e ail S S k NORSE PEAK 1732m Spukwush TONE CLIFF Bridge N Tolmie Peak t C A u r Redstone R 5939ft s Alice e G p e Peak C 1810m I Falls k re BEAR e Norse Peak k WILDERNESS Eunice -

Outings-2015.Pdf
2/11/2018 Sierra Club Activities Saturday, January 03, 2015 to Sunday, January 04, 2015 0452-Angeles Chp Hundred Peaks Outing CANCELLED RESCHEDULED TO APR 18 - 19 - I: Pahrump Point (5,740'), Stewart Point (5,265') Mat Kelliher 818-667-2490 [email protected] Bill Simpson 323-683-0959 [email protected] I: Pahrump Point (5,740'), Stewart Point (5,265') - Start out the New Year with a fun weekend of rocky peakbagging near Death Valley NP high above the Chicago Valley north-northeast of Shoshone, CA. We'll move at a slow pace each day; however, each peak will require a strenuous effort, and although the routes will be restricted to Class 2 scrambling, comfort on steep and loose, rocky and thorny cross-country terrain is required. Saturday morning we'll get an early start and head into the "Nopah Range" Wilderness Area located along the eastern range bordering Chicago Valley; first we'll warm up by trudging across a broad alluvial fan, then we'll make our way up through a sometimes tight and rocky canyon before getting up onto a steep and loose, rocky and thorny ridgeline that will bring us up onto the narrow and rocky summit ridge, which we'll ascend to the summit of Pahrump Point. After thoroughly enjoying the reportedly exquisite views up there, we'll return the way we came in for a day's total of 8 RT mi with 3,400' gain. We'll make camp where we're parked and will celebrate the weekend under a nearly full moon sky with a traditional DPS Potluck Happy Hour. -

Seattle and Then Later with Some Friends
WWW.MOUNTAINEERS.ORG NOVEMBER/DECEMBER 2014 • VOLUME 108 • NO. 6 Celebrating 50 years of Wilderness in the Pacific Northwest MountaineerEXPLORE • LEARN • CONSERVE 50 Years of the Wilderness Act PAGE 20 Meany Lodge a Gateway to Adventure PAGE 15 Gene’s Quest for 100 peaks at Mount Rainier PAGE 26 tableofcontents Nov/Dec 2014 » Volume 108 » Number 6 The Mountaineers enriches lives and communities by helping people explore, conserve, learn about and enjoy the lands and waters of the Pacific Northwest and beyond. Features 14 Meany Lodge a Gateway to Adventure 18 We All begin Somewhere from fear to leader 26 Gene’s Quest for 100 14 friendship and adventure on Rainier Columns 8 MEMBER highLight Patrick Mullaney 9 OUTDOOR EDUCation First Skis 10 IMPACT GIVING Small Gifts go a Long Way 12 TraiL Talk Trails Loved to Death? 20 CONSERVation Currents 50 Years of Wilderness 25 Natures WAY Life Rises From Ash 18 30 RETRO RewinD Wolf Bauer - a true pioneer Discover The Mountaineers Mountaineer magazine would like to thank The Mountaineers If you are thinking of joining — or have joined and aren’t sure where Foundation for its financial assistance. The Foundation operates to start — why not set a date to Meet The Mountaineers? Check the as a separate organization from The Mountaineers, which has received about one-third of the Foundation’s gifts to various Branching Out section of the magazine for times and locations of nonprofit organizations. informational meetings at each of our seven branches. Mountaineer uses: CLEAR on the cover: Liz Johnson rappels off Unicorn Peak in Mount Rainier National Park, led by climb leader, Ida Vincent. -

(In Two Parts) Part
UNITED STATES DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY EVALUATION OF THE WILKESON-CARBONADO COAL FIELD, PIERCE COUNTY, WASHINGTON FOR HYDRAULIC COAL MINING (In Two Parts) Part One - Geology Part Two - Water Resources Prepared in cooperation with the Bureau of Mines This report has not been edited or reviewed for co.nformity with U.S. Geological Survey standards or nomenclature. Open File Report 80-802 EVALUATION OF THE WILKESON-CARBONADO COAL FIELD, PIERCE COUNTY, WASHINGTON FOR HYDRAULIC COAL MINING Part one - Geology GEOLOGIC EVALUATION OF THE WILKESON-CARBONADO COAL FIELD, PIERCE COUNTY, WASHINGTON, FOR HYDRAULIC COAL MINING By Harold H. Arndt and Bion H. Kent Prepared by the Branch of Coal Resources, Denver, Colorado, 1977 CONTENTS Page Summary———————————————————————————————"—————————————— 1 Introduction—•————————————————;—————————————————————— 2 Scope of investigations————————————————————————————— 2 Location and geologic setting——————————————————————— 3 Previous investigations————————————————-———————————— 7 Data acquisition and acknowledgements—————————————————— 8 Geography-—————————————————————:—————————————————————— . 9 Topography and drainage————————————————————————————— 9 Climate and vegetation————————————————————————————— 11 Population and ownership————————————————————————————— 12 Accessibility———•—————————————————————————————————— 15 Stratigraphy—————————-———————————————————————————————— 15 General features——————————————————————————————————- 15 Tertiary System———————————————————————————————————— 15 Eocene Series————————————————————————————————— -

Get Ting to Know the Mountain a Wonderland Trail Journal
News & Features Getting to Know the Mountain ARNOSKI B AURIE L In July 2005, two brothers, Mike and Steve, embarked on a once-in-a-lifetime trip: completing the 93-mile Wonderland Trail around Mount Rainier. Above: Steve at Narada Falls. Right: hikers near Carbon River Glacier. A Wonderland Trail Journal By “Mike in Tac” hitting the road. After a couple of stops them and shared their space for 5 or 10 for errands and phone calls, we got our minutes. They never ran off—we finally permit and arrived here. left them! Thursday, July 7, 2005, 10:22 p.m. We wandered up to Eunice Lake and Back to the tent at 7:00 p.m. and we Mowich Lake Campground, fished a bit—no views, clouds totally raided the “free pile” of food at the 5,000 feet socked us in. I had terrible luck, lost ranger cabin for dinner. Lots of cocoa Overcast, fogged in, 74 degrees in a lure (Steve’s) on the first cast. Steve too. Met Tim—another guy (with two tent, 60 degrees outside had much better luck with his $7.50 friends—Joan and Todd, I think)—who Slept in a bit and said good-bye to Jane rod and reel. On the way back, we ran will start the Wonderland tomorrow as at 8:00 a.m. Steve and I then packed for a in to a couple of one-year-old bucks—no well. They’re doing an 8-day trip. couple of hours before having lunch and fear whatsoever. -

State of Washington
DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, DIBECTOR BULLETIN 557 STATE OF WASHINGTON 1896 TO 1913, INCLUSIVE R. B. MARSHALL, CHIEF GEOGRAPHER WORK FROM 1909 TO 1913, INCLUSIVE, DONE IN COOPERATION WITH THE STATE HENRY LANDES, STATE GEOLOGIST WASHINGTON GOVERNMENT PRINTING OFFICE 1914 ELEVATION OF MOUNT BAINIEB. The elevation of the highest snow-capped summit of Mount Rainier was determined by C. H. Birdseye in 1913 from carefully checked vertical angles based on spirit-level elevation of McClure Rock. The distance between McClure Rock and Crater, a station marked by a rock cairn on the bare south rim of the mountain, was computed from triangulation data. The distance from Crater to the summit, which is 980 feet, was measured by stadia and checked by plane-table intersections. The elevation of Crater is 14,267 feet and that of the summit 14,408 feet. Mount Rainier is, so far as known, the second highest point in the United States. CONTENTS. Page. Introduction............................................................... 5 Cooperation..........................-...............:.................. 5 Previous publication.................................................. 5 Personnel............................................................. 5 Classification.......................................................... 5 Bench marks........................................................... 6 Datum............................................................... 6 Topographic maps..................................................... -
MOUNT RAINIER NATIONAL PARK Ier C S D in H O St
CLEARWATER k e re WILDERNESS C Carbon River Road subject to closure r e e due to river flooding o v MT. BAKER-SNOQUALMIE NATIONAL FOREST D i R To Wilkeson Ca iver 13mi / 21km from rbon arbon R Road Carbon River Entrance C r e r 4mi v e e 6km C i t G E v h i i Carbon River Entrance Chenuis Falls R 410 o G e Lake R h a 1880ft k e t n D I t Eleanor e 573m u i W e R J r Tirzah Peak i h k s u e C 5208ft n re Adelaide Pigeon Peak W Scarface k e L Obtain Climbing and Wilderness 1587m E e C k 6108ft o C C Lake r re re Wallace Peak Oliver 1862m s e N A k Camping Permits for the northwest o C t Sweet C Ranger Falls E F d C E H Lake a area of the park at Carbon River Peak r G E D e N t s U N s e l D e Ranger Station. l I E k a I S Marjorie C P Slide Mountain F R W 2749ft r E C N46° 58´ 42˝ Lake e 6339ft M r Green Ipsut Creek D 838m e W121 32´ 07˝ T O e 1932m ° N k e Lake U I S r Chenuis Florence Peak N y k e r 5508ft U Ca T r g r A r A R b B Lakes C e 1679m n o EE I n L n N r b K a FL o e Gove Peak A Lake l I R T H C S k i r l C NORTH 5310ft Ethel n c v Arthur Peak J e R 1619m a u o e E e r NORSE PARK 5483ft W R V H se S iv k T 1671m on e ph d r K i e Lake R n BURNT C rl Tyee A e r an YE James P PARK e d LL Peak e PEAK O Natural k Howard Peak sut WS D Ip Tr Spukwus TONE CLIFFS Bridge N 5683ft Tolmie Peak ail h C A 1732m r R 5939ft Alice e Redstone G e C Norse Peak WILDERNESS 1810m Falls k Peak re BEAR ek 6856ft Eunice Lake TH PARK k 2090m Cress E e August Windy PAL e 5mi Falls Crescent ISAD r Peak Castle Peak N Gap ES C 8km 6110ft I Lake A IN GREEN -

Mount Rainier National Park Place Names
Mount Rainier National Park Place Names Gary Fuller Reese. April 10, 2009. PREFACE. Because of its prominence as the "Great Mountain of the Pacific Northwest" Rainier was one of the first features in the Pacific Northwest named by early explorers. The center of a National Park since 1899 most prominent features around the mountain have received names, some of which have become official and some of which a common use. In 1919 Mount Rainier National Park Superintendent Roger Toll wrote about names in the National Park: "The park service is interested in having names applied to the various...scenic points that are now unnamed....the most desirable names...are the original Indian names, or, if these are too long and unpronounceable their English equivalents are often very good. "If no original name can be found, and a name is to be supplied, the Indian names may be drawn upon with advantage, but this should be done by an expert...Descriptive names are good. The only thing most difficult to avoid is the indiscriminate naming of scenic features after persons." While name origins have been found for many locations within the National Park there are a number of places for which origins are missing, especially on the northern side of the mountain. In 1916 Edmond S. Meany wrote about Mount Rainier. He listed many places for which he could not find a name origin. In 1932 the writers of the Encyclopedia of Information on Mount Rainier National Park made a list of locations on the mountain recording that they were unable to supply origins for numbers of them. -

Summits on the Air USA (W7W)
Summits on the Air U.S.A. (W7W) Association Reference Manual (ARM) Document Reference S39.1 Issue number 2.0 Date of issue 01-Dec-2016 Participation start date 01-July-2009 Authorised Date 08-Jul-2009 obo SOTA Management Team Association Manager Darryl Holman, WW7D, [email protected] Summits-on-the-Air an original concept by G3WGV and developed with G3CWI Notice “Summits on the Air” SOTA and the SOTA logo are trademarks of the Programme. This document is copyright of the Programme. All other trademarks and copyrights referenced herein are acknowledged. Summits on the Air – ARM for USA W7W-Washington Table of contents Change Control ................................................................................................................... 4 Disclaimer ........................................................................................................................... 5 Copyright Notices ............................................................................................................... 5 1.0 Association Reference Data .......................................................................................... 6 2.1 Program Derivation ....................................................................................................... 7 2.2 General Information ...................................................................................................... 7 2.3 Final Access, Activation Zone, and Operating Location Explained ............................. 8 2.4 Rights of Way and Access Issues ................................................................................