Petroleum Hydrocarbon Gases Category Analysis and Hazard Characterization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Disability Classification System
CLASSIFICATION SYSTEM FOR STUDENTS WITH A DISABILITY Track & Field (NB: also used for Cross Country where applicable) Current Previous Definition Classification Classification Deaf (Track & Field Events) T/F 01 HI 55db loss on the average at 500, 1000 and 2000Hz in the better Equivalent to Au2 ear Visually Impaired T/F 11 B1 From no light perception at all in either eye, up to and including the ability to perceive light; inability to recognise objects or contours in any direction and at any distance. T/F 12 B2 Ability to recognise objects up to a distance of 2 metres ie below 2/60 and/or visual field of less than five (5) degrees. T/F13 B3 Can recognise contours between 2 and 6 metres away ie 2/60- 6/60 and visual field of more than five (5) degrees and less than twenty (20) degrees. Intellectually Disabled T/F 20 ID Intellectually disabled. The athlete’s intellectual functioning is 75 or below. Limitations in two or more of the following adaptive skill areas; communication, self-care; home living, social skills, community use, self direction, health and safety, functional academics, leisure and work. They must have acquired their condition before age 18. Cerebral Palsy C2 Upper Severe to moderate quadriplegia. Upper extremity events are Wheelchair performed by pushing the wheelchair with one or two arms and the wheelchair propulsion is restricted due to poor control. Upper extremity athletes have limited control of movements, but are able to produce some semblance of throwing motion. T/F 33 C3 Wheelchair Moderate quadriplegia. Fair functional strength and moderate problems in upper extremities and torso. -

IPC Accessibility Guide
2 TABLE OF CONTENTS FIGURES AND TABLES ................................................................................................................. 8 Foreword ........................................................................................................................................... 10 Introduction ................................................................................................................................. 10 Evolving content ......................................................................................................................... 10 Disclosure ...................................................................................................................................... 11 Structure and content of the IPC Accessibility Guide ...................................................... 11 Content ........................................................................................................................................... 11 Executive summary ......................................................................................................................... 12 Aim and purpose of the Guide ................................................................................................ 12 Key objectives of the Guide ..................................................................................................... 12 Target audience of the Guide ................................................................................................. 12 1 General information -

Ifds Functional Classification System & Procedures
IFDS FUNCTIONAL CLASSIFICATION SYSTEM & PROCEDURES MANUAL 2009 - 2012 Effective – 1 January 2009 Originally Published – March 2009 IFDS, C/o ISAF UK Ltd, Ariadne House, Town Quay, Southampton, Hampshire, SO14 2AQ, GREAT BRITAIN Tel. +44 2380 635111 Fax. +44 2380 635789 Email: [email protected] Web: www.sailing.org/disabled 1 Contents Page Introduction 5 Part A – Functional Classification System Rules for Sailors A1 General Overview and Sailor Evaluation 6 A1.1 Purpose 6 A1.2 Sailing Functions 6 A1.3 Ranking of Functional Limitations 6 A1.4 Eligibility for Competition 6 A1.5 Minimum Disability 7 A2 IFDS Class and Status 8 A2.1 Class 8 A2.2 Class Status 8 A2.3 Master List 10 A3 Classification Procedure 10 A3.0 Classification Administration Fee 10 A3.1 Personal Assistive Devices 10 A3.2 Medical Documentation 11 A3.3 Sailors’ Responsibility for Classification Evaluation 11 A3.4 Sailor Presentation for Classification Evaluation 12 A3.5 Method of Assessment 12 A3.6 Deciding the Class 14 A4 Failure to attend/Non Co-operation/Misrepresentation 16 A4.1 Sailor Failure to Attend Evaluation 16 A4.2 Non Co-operation during Evaluation 16 A4.3 International Misrepresentation of Skills and/or Abilities 17 A4.4 Consequences for Sailor Support Personnel 18 A4.5 Consequences for Teams 18 A5 Specific Rules for Boat Classes 18 A5.1 Paralympic Boat Classes 18 A5.2 Non-Paralympic Boat Classes 19 Part B – Protest and Appeals B1 Protest 20 B1.1 General Principles 20 B1.2 Class Status and Protest Opportunities 21 B1.3 Parties who may submit a Classification Protest -

Isobutylene Polymer Having Unsaturated Group and Preparation
Europaisches Patentamt European Patent Office Office europeen des brevets (11) EP 0 452 875 B2 (12) NEW EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) |nt CI.6: C08F 10/10, C08F 2/42 of the opposition decision: 18.08.1999 Bulletin 1999/33 (45) Mention of the grant of the patent: 09.08.1995 Bulletin 1995/32 (21) Application number: 91106042.4 (22) Date of filing: 16.04.1991 (54) Isobutylene polymer having unsaturated group and preparation thereof Isobutylenpolymer mit ungesattigter Gruppe und seine Herstellung Polymere de I'isobutylene ayant un groupe insature et sa preparation (84) Designated Contracting States: (74) Representative: BE CH DE ES FR GB IT LI NL SE Hansen, Bernd, Dr. Dipl.-Chem. et al Hoffmann Eitle, (30) Priority: 16.04.1990 JP 10102990 Patent- und Rechtsanwalte, 29.11.1990 JP 33636490 Postfach 81 04 20 81904 Miinchen (DE) (43) Date of publication of application: 23.10.1991 Bulletin 1991/43 (56) References cited: EP-A- 0 265 053 EP-B- 0 322 241 (73) Proprietor: Kanegafuchi Chemical Industry Co., CA-A- 467 049 GB-A- 1 059 580 Ltd. US-A- 4 31 6 973 US-A- 4 327 201 Kita-ku Osaka-shi Osaka-fu (JP) US- A- 4 524 1 88 US-A- 4 758 63 1 US-A- 4 929 683 (72) Inventors: • Noda, Koji Remarks: Kobe-shi, Hyogo-ken (JP) The file contains technical information submitted • Fujisawa, Hiroshi after the application was filed and not included in this Kobe-shi, Hyogo-ken (JP) specification • Yonezawa, Kazuya Kobe-shi, Hyogo-ken (JP) CM DO 10 Is- 00 CM IO ^- o a. -

Model Vulcanization Systems for Butyl Rubber and Halobutyl Rubber Manual
Exxon™ butyl and halobutyl rubber Model vulcanization systems for butyl rubber and halobutyl rubber manual Country name(s) 2 - Model vulcanization systems for butyl rubber and halobutyl rubber manual Model vulcanization systems for butyl rubber and halobutyl rubber manual - 3 Abstract The vulcanization of isobutylene-co-isoprene rubber (IIR), brominated isobutylene-co-isoprene rubber (BIIR), chlorinated isobutylene-co-isoprene rubber (CIIR), and brominated isobutylene-co-para-methylstyrene elastomer (BIMSM) differs from that of general-purpose rubbers (GPR). Butyl rubber has approximately 2% unsaturation in the backbone. Halobutyl rubber (BIIR and CIIR) incorporates the butyl backbone with either bromine or chlorine, which significantly increases the chemical reactivity of the isoprenyl units located in the butyl backbone. Similarly, in BIMSM the bromine atom is bonded to the para-methylstyrene (PMS) group, thus affording the completely saturated polymer backbone a site of chemical reactivity. Utilization of the unique attributes of butyl rubber and halobutyl rubbers with their minimal backbone unsaturation and of BIMSM elastomers with no backbone unsaturation is found in many areas of industry. These properties are excellent vapor impermeation, resistance to heat degradation, and improved chemical resistance as compared to general-purpose rubbers. However, this low amount of reactivity requires special consideration to vulcanize these isobutylene-based polymers. The type of vulcanization system selected is a function of the composite structure in which it is used, and the cured product performance requirements. Therefore, vulcanization systems vary and may include an accelerator package along with resins, zinc oxide, zinc oxide and sulfur, and quinoid systems. This review will discuss the types and selection of appropriate vulcanization systems for isobutylene-based elastomers. -

(HDS) Unit for Petroleum Naphtha at 3500 Barrels Per Day
Available online at www.worldscientificnews.com WSN 9 (2015) 88-100 EISSN 2392-2192 Design Parameters for a Hydro desulfurization (HDS) Unit for Petroleum Naphtha at 3500 Barrels per Day Debajyoti Bose University of Petroleum & Energy Studies, College of Engineering Studies, P.O. Bidholi via- Prem Nagar, Dehradun 248007, India E-mail address: [email protected] ABSTRACT The present work reviews the setting up of a hydrodesulphurization unit for petroleum naphtha. Estimating all the properties of the given petroleum fraction including its density, viscosity and other parameters. The process flow sheet which gives the idea of necessary equipment to be installed, then performing all material and energy balance calculations along with chemical and mechanical design for the entire setup taking into account every instrument considered. The purpose of this review paper takes involves an industrial process, a catalytic chemical process widely used to remove sulfur (S) from naphtha. Keywords: hydro desulfurization, naphtha, petroleum, sulfur Relevance to Design Practice - The purpose of removing the sulfur is to reduce the sulfur dioxide emissions that result from using those fuels in automotive vehicles, aircraft, railroad locomotives, gas or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion. World Scientific News 9 (2015) 88-100 1. INTRODUCTION Hydrodesulphurization (HDS) is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of removing the sulfur is to reduce the sulfur dioxide (SO2) emissions that result from various combustion practices. -

Material Safety Data Sheet
MATERIAL NAME: Heavy Straight MSDS # EPL-13 Run Naphtha MATERIAL SAFETY DATA SHEET SECTION 1 X PRODUCT AND COMPANY IDENTIFICATION FOR EMERGENCY SOURCE INFORMATION CONTACT: Explorer Pipeline Company ¾ (918) 493 - 5100 6846 South Canton ¾ CHEMTREC: (800) 424-9300 (24 hour contact) P.O. Box 2650 ¾ CANUTEC: (613) 996-6666 Tulsa, Oklahoma 74101 ¾ SETIQ: 91-800-00214 TRADE NAMES/SYNONYMS: Penex CHEMICAL FAMILY: Mixture of C - 4 EPL Code: 10 Raffinate C6, Hydrocarbons This material safety data sheet represents the composite characteristics and properties of fungible petroleum hydrocarbons and other related substances transported by explorer pipeline company. The information presented was compiled from one or more product shipper sources and is intended to provide health and safety guidance for these fungible products. Individual shipper and manufacturer MSDSs are available at Explorer Pipeline Company’s, Tulsa, Oklahoma, offices. SECTION 2 ; HAZARDS IDENTIFICATION )))))))))))))))))EMERGENCY OVERVIEW))))))))))))))))) Flammable Liquid!! ¾ Clear liquid with petroleum odor; ¾ Harmful or fatal if swallowed, inhaled or absorbed through skin. ¾ May cause CNS depression. ¾ Can produce skin irritation upon prolonged or repeated contact. ¾ Keep away from heat, sparks and open flame; ¾ Wash thoroughly after handling; ¾ Contains petroleum distillates! If swallowed, do not induce vomiting since aspiration into the lungs will cause chemical pneumonia; ¾ Avoid breathing vapors or mist; ¾ Use only with adequate ventilation; and ¾ Obtain prompt medical attention. Keep Out of Reach of Children! )))))))))))))))))))))))))))))))))))))))))))))) SECTION 3 W COMPOSITION/INFORMATION OF INGREDIENTS INGREDIENT CAS NUMBER PERCENTAGE (%) Mixture of C4-C6 hydrocarbons 64741-70-4 100% ACUTE SUMMARY OF ACUTE HAZARDS: Harmful or fatal if swallowed, inhaled or absorbed through the skin. May cause CNS depression. -

FID Vs PID: the Great Debate
5/5/2017 FID vs PID: The Great Debate 2017 Geotechnical, GeoEnvironmental, and Geophysical Technology Transfer April 11, 2017 Raleigh, NC Why is it Important? As consultants, we are expected to produce data that is Reliable Repeatable Representative Defensible We can only meet these criteria if we understand the instruments we use and their limitations 1 5/5/2017 PID=Photo Ionization Detector Non-destructive to the sample Responds to functional groups Can operate in non-oxygen atmosphere Does not respond to methane Affected by high humidity FID=Flame Ionization Detector Destructive to the sample Responds to carbon chain length Must have oxygen to operate Responds to methane Not affected by high humidity 2 5/5/2017 Combination FID/PID TVA 1000B Main Concepts Ionization Energy Minimum amount of energy required to remove an electron from an atom or molecule in a gaseous state Response Factors The response factor is a calculated number provided by the instrument manufacturer for each compound, which is used to calculate the actual concentration of said compound in relation to the calibration gas. 3 5/5/2017 Ionization Energy Basis for FID/PID operations and measurement Measurements are in electron volts (eV) Ionization in a PID Energy source for ionization with PID is an ultraviolet light Three UV lamp energies are used: 9.5 eV, 10.6 eV, and 11.7 eV The higher the lamp energy, the greater the number of chemicals that can be detected. Detection range of 0.1 to 10,000 ppm 4 5/5/2017 Ionization in a FID Energy source for ionization -
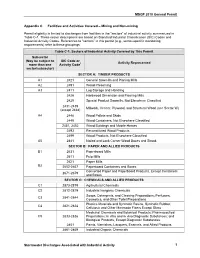
Facility SIC Code Table
MSGP 2010 General Permit Appendix C. Facilities and Activities Covered – Mining and Non-mining Permit eligibility is limited to discharges from facilities in the “sectors” of industrial activity summarized in Table C-1. These sector descriptions are based on Standard Industrial Classification (SIC) Codes and Industrial Activity Codes. References to “sectors” in this permit (e.g., sector-specific monitoring requirements) refer to these groupings. Table C-1. Sectors of Industrial Activity Covered by This Permit Subsector (May be subject to SIC Code or Activity Represented more than one Activity Code1 sector/subsector) SECTOR A: TIMBER PRODUCTS A1 2421 General Sawmills and Planing Mills A2 2491 Wood Preserving A3 2411 Log Storage and Handling 2426 Hardwood Dimension and Flooring Mills 2429 Special Product Sawmills, Not Elsewhere Classified 2431-2439 Millwork, Veneer, Plywood, and Structural Wood (see Sector W) (except 2434) A4 2448 Wood Pallets and Skids 2449 Wood Containers, Not Elsewhere Classified 2451, 2452 Wood Buildings and Mobile Homes 2493 Reconstituted Wood Products 2499 Wood Products, Not Elsewhere Classified A5 2441 Nailed and Lock Corner Wood Boxes and Shook SECTOR B: PAPER AND ALLIED PRODUCTS B1 2631 Paperboard Mills 2611 Pulp Mills 2621 Paper Mills B2 2652-2657 Paperboard Containers and Boxes Converted Paper and Paperboard Products, Except Containers 2671-2679 and Boxes SECTOR C: CHEMICALS AND ALLIED PRODUCTS C1 2873-2879 Agricultural Chemicals C2 2812-2819 Industrial Inorganic Chemicals Soaps, Detergents, and Cleaning Preparations; -

Platinum Catalysts in Petroleum Refining
Platinum Catalysts in Petroleum Refining By S. W. Curry, B.s., M.B.A. Universal Oil Products Company, Des Plnines, Illinois Rejbriiiirig processes using platinunz catalysts have beconie oj- major importance in petroleum rejining during the past seven years. The?. enable the octane rating of naphthas to be greatly increased, am1 cue more economicnl than any other rejiningyrocess for the production of high octane gasoline. In this article the general nature of the processes is described and the Platforniing process is considwed iti iiiore rletnil. Platinum in any form was virtually unused years. The end is by no means in sight, in the petroleum industry until 1949. Then since the trend in octane number requirement, Universal Oil Products Company introduced particularly for automobiles, has continued it on an unprecedented scale as the active to creep upward year by year. catalytic agent in its Platforming process for To have advocated the use of 400 ounces catalytically upgrading low octane petroleum of a noble metal, selling at about $70 per naphthas to high quality products. ounce at that time, in a catalyst charge for a Prior to the installation of the first UOP single small commercial refinery unit, would Platforming unit, platinum was found chiefly doubtless have been branded prior to 1949 in laboratories in the oil industry. In sharp as the impractical idea of a dreamer. UOP's contrast with 1949, platinum today may be announcement surprised many in the oil regarded as a most essential item in the pro- industry for that matter. duction of high octane gasoline for automo- Even after the first Platformer had been biles and piston-engine aircraft. -

&EPA Ambient Water Quality Criteria for Toluene
United States Cffice of Water EPA 440/5-80-075 Environmental Protection Regula.ions and Standards O,:tober 1980 Agency Criteria and Standards Division Washington DC 20480 c.). & EPA Ambient Water Quality Criteria for Toluene tz r AMBIENT WATER QUALITY CRITERIA FOR TOLUENE Prepared By U.S. ENVIRONMENTAL PROTECTION AGENCY Office of Water Regulations and Standards Criteria and Standards Division Washington, D.C. Office of Research and Development Environmental Criteria and Assessment Office Cincinnati, Ohio Carcinogen Assessment Group Washington, D.C. Environmental Research Laboratories Corvalis, Oregon Duluth, Minnesota Gulf Breeze, Florida Narragansett, Rhode Island DISCLAIMER This report has been reviewed by the Environmental Criteria and Assessment Office, U.S. Environmental Protection Agency, and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. AVAILABILITY NOTICE This document is available to the public through the National Technical Information Service, (NTIS), Springfield, Virginia 22161. El~TAL l'R~e1'!tjf A~ ii FOREWORD Section 304 (a)(1) of the Clear Water Act of 1977 (P.L. 95-217), requires the Administrator of the Environmental Protection Agency to publish criteria for water quality accurately reflecting the latest scientific knowledge on the kind and extent of all identifiable effects on health and welfare which may be expected from the presence of pollutants in any body of water, including ground water. Proposed water quality criteria for the 65 toxic pollutants listed under section 307 (a) (1) of the Cl ean Water Act were deve loped and a notice of thei r availability was published for public comment on March 15, 1979 (44 FR 15926), July 25, 1979 (44 FR 43660), and October 1, 1979 (44 FR 56628). -

Introduction to Alkenes and Alkynes in an Alkane, All Covalent Bonds
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene, however, only three σ bonds are formed from the alkene carbon -the carbon thus adopts an sp2 hybridization Ethene (common name ethylene) has a molecular formula of CH2CH2 Each carbon is sp2 hybridized with a σ bond to two hydrogens and the other carbon Hybridized orbital allows stronger bonds due to more overlap H H C C H H Structure of Ethylene In addition to the σ framework of ethylene, each carbon has an atomic p orbital not used in hybridization The two p orbitals (each with one electron) overlap to form a π bond (p bonds are not symmetric about the internuclear axis) π bonds are not as strong as σ bonds (in ethylene, the σ bond is ~90 Kcal/mol and the π bond is ~66 Kcal/mol) Thus while σ bonds are stable and very few reactions occur with C-C bonds, π bonds are much more reactive and many reactions occur with C=C π bonds Nomenclature of Alkenes August Wilhelm Hofmann’s attempt for systematic hydrocarbon nomenclature (1866) Attempted to use a systematic name by naming all possible structures with 4 carbons Quartane a alkane C4H10 Quartyl C4H9 Quartene e alkene C4H8 Quartenyl C4H7 Quartine i alkine → alkyne C4H6 Quartinyl C4H5 Quartone o C4H4 Quartonyl C4H3 Quartune u C4H2 Quartunyl C4H1 Wanted to use Quart from the Latin for 4 – this method was not embraced and BUT has remained Used English order of vowels, however, to name the groups