Retention and Transmission of Grapevine Leafroll-Associated Virus 3 by Pseudococcus Calceolariae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Population Dynamic of the Long-Tailed
Assiut J. of Agric. Sci., 42 No.(5) (143-164) Population Dynamic of the Long-tailed Mealybug, Pseudococcus longispinus (Targioni-Tozzetti) Infest- ing the Ornamental Plant, Acalypha marginata Green, under Assiut governorate conditions. Ghada,S.Mohamed1; Abou-Ghadir,M.F.2; Abou- Elhagag,G.H.2 and Gamal H. Sewify3 1Dept. of plant protec., Fac. Agric., South Valley Univ. 2Dept. of plant protec., Fac. Agric., Assiut Univ. 3Dept. of plant protec., Fac. Agric., Cairo Univ. Abstract centages of parasitism ranged The shrubs of ornamental from 0.01 in January to 0.06% in plant were inspected as host of March and 0.007 to 0.05% in the the studied pest. The present same months during the first and study was carried out in the Ag- the second season of study. The riculture Experimental Station of seasonal abundance of this para- the Faculty of Agriculture, Assiut sitoid species and the effect of university, during two successive weather elements on its popula- seasons of 2008/2009 and tion were also studied. 2009/2010. Results of both sea- Introduction sons showed that the highest The ornamental plant, Aca- weekly population count of the lypha marginata is a common mealybug, Pesudococcus long- shrub planted for decoration ispinus (Targioni-Tozzetti) was along the streets. This plant is found during the 2rd half of Au- susceptible to the mealybug in- gust. The highest percentage of festation that cause a serious mal- the total monthly mean count was formation to its leaves. The also recorded during August common name of the mealy bugs (30% out of the total year is derived from the mealy wax count).The pest has four genera- secretion that usually covers their tions in each of the tow studied bodies (Kosztarab, 1996). -

An Investigation Into the Integrated Pest Management of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Stellenbosch University SUNScholar Repository An investigation into the integrated pest management of the obscure mealybug, Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae), in pome fruit orchards in the Western Cape Province, South Africa. Pride Mudavanhu Thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Agriculture (Entomology), in the Faculty of AgriSciences. at the University of Stellenbosch Supervisor: Dr Pia Addison Department of Conservation Ecology and Entomology Faculty of AgriSciences University of Stellenbosch South Africa December 2009 DECLARATION By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the owner of the copyright thereof (unless to the extent explicitly otherwise stated) and that I have not previously in its entirety or in part submitted it for obtaining any qualification. December 2009 Copyright © 2009 Stellenbosch University All rights reserved i ABSTRACT Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae) (obscure mealybug), is a common and serious pest of apples and pears in South Africa. Consumer and regulatory pressure to produce commodities under sustainable and ecologically compatible conditions has rendered chemical control options increasingly limited. Information on the seasonal occurrence of pests is but one of the vital components of an effective and sustainable integrated pest management system needed for planning the initiation of monitoring and determining when damage can be expected. It is also important to identify which orchards are at risk of developing mealybug infestations while development of effective and early monitoring tools for mealybug populations will help growers in making decisions with regards to pest management and crop suitability for various markets. -

Transmission of Grapevine Leafroll-Associated Virus 3 (Glrav-3): Acquisition, Inoculation and Retention by the Mealybugs Planococcus Ficus and Pseudococcus Longispinus (Hemiptera
Transmission of Grapevine Leafroll-associated Virus 3 (GLRaV-3): Acquisition, Inoculation and Retention by the Mealybugs Planococcus ficus and Pseudococcus longispinus (Hemiptera: Pseudococcidae) K. Krüger1,*, D.L. Saccaggi1,3, M. van der Merwe2, G.G.F. Kasdorf2 (1) Department of Zoology & Entomology, University of Pretoria, Private Bag X20, Pretoria, 0028, South Africa (2) ARC-Plant Protection Research Institute, Private Bag X134, Pretoria, 0001, South Africa (3) Current address: Plant Health Diagnostic Services, Department of Agriculture, Forestry and Fisheries, Private Bag X5015, Stellenbosch, 7599, South Africa Submitted for publication: December 2014 Accepted for publication: March 2015 Key words: Ampelovirus, Closteroviridae, Coccoidea, grapevine leafroll disease, Vitis vinifera The vine mealybug, Planococcus ficus (Signoret), and the longtailed mealybug, Pseudococcus longispinus (Targioni Tozzetti), are vectors of grapevine leafroll-associated virus 3 (GRLaV-3), one of the most abundant viruses associated with grapevine leafroll disease. To elucidate the transmission biology in South Africa, acquisition access periods (AAPs), inoculation access periods (IAPs) and the retention of the virus in starving and feeding first- to second instar nymphs were determined. The rootstock hybrid LN33 served as virus source and grapevines (Vitis vinifera L., cv. Cabernet franc) served as recipient plants. An AAP of 15 min or an IAP of 15 min was sufficient forPl. ficus to acquire or transmit GLRaV-3, respectively. Nymphs of Pl. ficus retained the virus for at least eight days when feeding on a non-virus host and grapevine, and for at least two days when starving, and were then capable of transmitting it successfully to healthy grapevine plants. Nymphs of Ps. longispinus transmitted the virus after an AAP of 30 min and an IAP of 1 h. -
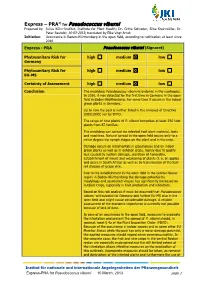
Express PRA Pseudococcus Viburni
1) Express – PRA for Pseudococcus viburni Prepared by: Julius Kühn-Institut, Institute for Plant Health; Dr. Gritta Schrader, Silke Steinmöller, Dr. Peter Baufeld; 10-03-2013; translated by Elke Vogt-Arndt Initiation: Occurrence in Baden-Württemberg in the open field, according to notification at least since 2010 Express - PRA Pseudococcus viburni (Signoret) Phytosanitary Risk for high medium low Germany Phytosanitary Risk for high medium low EU-MS Certainty of Assessment high medium low Conclusion The mealybug Pseudococcus viburni is endemic in the neotropics. In 2010, it was detected for the first time in Germany in the open field in Baden-Württemberg. For some time it occurs in the indoor green plants in Germany. Up to now the pest is neither listed in the annexes of Directive 2000/29/EC nor by EPPO. The range of host plants of P. viburni comprises at least 296 host plants from 87 families. The mealybug can spread via infested host plant material, tools and machines. Natural spread in the open field occurs only to a minor degree via nymph stages on the plant and in the crop. Damage occurs on ornamentals in glasshouses and on indoor green plants as well as in outdoor crops, mainly due to quality loss caused by suction damage, secretion of honeydew, establishment of mould and weakening of plants (f. e. on apples and pears in South Africa) as well as by transmission of the leaf- roll disease of grape vine. Due to the establishment in the open field in the central Neckar region in Baden-Württemberg the damage potential by mealybugs and associated viruses has significantly increased on outdoor crops, especially in fruit production and viticulture. -

Checklist of the Scale Insects (Hemiptera : Sternorrhyncha : Coccomorpha) of New Caledonia
Checklist of the scale insects (Hemiptera: Sternorrhyncha: Coccomorpha) of New Caledonia Christian MILLE Institut agronomique néo-calédonien, IAC, Axe 1, Station de Recherches fruitières de Pocquereux, Laboratoire d’Entomologie appliquée, BP 32, 98880 La Foa (New Caledonia) [email protected] Rosa C. HENDERSON† Landcare Research, Private Bag 92170 Auckland Mail Centre, Auckland 1142 (New Zealand) Sylvie CAZÈRES Institut agronomique néo-calédonien, IAC, Axe 1, Station de Recherches fruitières de Pocquereux, Laboratoire d’Entomologie appliquée, BP 32, 98880 La Foa (New Caledonia) [email protected] Hervé JOURDAN Institut méditerranéen de Biodiversité et d’Écologie marine et continentale (IMBE), Aix-Marseille Université, UMR CNRS IRD Université d’Avignon, UMR 237 IRD, Centre IRD Nouméa, BP A5, 98848 Nouméa cedex (New Caledonia) [email protected] Published on 24 June 2016 Rosa Henderson† left us unexpectedly on 13th December 2012. Rosa made all our recent c occoid identifications and trained one of us (SC) in Hemiptera Sternorrhyncha slide preparation and identification. The idea of publishing this article was largely hers. Thus we dedicate this article to our late and dear Rosa. Rosa Henderson† nous a quittés prématurément le 13 décembre 2012. Rosa avait réalisé toutes les récentes identifications de cochenilles et avait formé l’une d’entre nous (SC) à la préparation des Hemiptères Sternorrhynques entre lame et lamelle. Grâce à elle, l’idée de publier cet article a pu se concrétiser. Nous dédicaçons cet article à notre chère et regrettée Rosa. urn:lsid:zoobank.org:pub:90DC5B79-725D-46E2-B31E-4DBC65BCD01F Mille C., Henderson R. C.†, Cazères S. & Jourdan H. 2016. — Checklist of the scale insects (Hemiptera: Sternorrhyncha: Coccomorpha) of New Caledonia. -

Hemiptera: Pseudococcidae) Diversity in Received: 5 April 2017 Accepted: 1 November 2017 Southern Brazilian Fruit Crops Published: Xx Xx Xxxx Vitor C
www.nature.com/scientificreports OPEN Integrative taxonomy methods reveal high mealybug (Hemiptera: Pseudococcidae) diversity in Received: 5 April 2017 Accepted: 1 November 2017 southern Brazilian fruit crops Published: xx xx xxxx Vitor C. Pacheco da Silva1, Mehmet Bora Kaydan2,3, Thibaut Malausa4, Jean-François Germain 5, Ferran Palero4,6 & Marcos Botton7 The Serra Gaúcha region is the most important temperate fruit-producing area in southern Brazil. Despite mealybugs (Hemiptera: Pseudococcidae) infesting several host plants in the region, there is a lack of information about the composition of species damaging diferent crops. A survey of mealybug species associated with commercial fruit crops (apple, persimmon, strawberry and grapes) was performed in Serra Gaúcha between 2013 and 2015, using both morphology and DNA analyses for species identifcation. The most abundant species were Pseudococcus viburni (Signoret), found on all four host plant species, and Dysmicoccus brevipes (Cockerell), infesting persimmon, vines and weeds. The highest diversity of mealybug species was found on persimmon trees, hosting 20 diferent taxa, of which Anisococcus granarae Pacheco da Silva & Kaydan, D. brevipes, Pseudococcus sociabilis Hambleton and Ps. viburni were the most abundant. A total of nine species were recorded in vineyards. Planococcus fcus (Signoret) and Pseudococcus longispinus (Targioni Tozzetti) were observed causing damage to grapes for the frst time. A single species, Ps. viburni, was found associated with apples, while both Ps. viburni and Ferrisia meridionalis Williams were found on strawberry. Four of the mealybug species found represent new records for Brazil. Brazil is the third largest fruit producer in the world, with a cultivated area of 2.5 million hectares and an esti- mated production of 40 million tons1,2. -

Florida Coonties and Atala Butterflies1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. ENH117 Florida Coonties and Atala Butterflies1 Daniel F. Culbert2 Sunshine State gardeners have rediscovered the Coontie Relatives Florida coontie (Figure 1) as a native plant well adapted to Florida yards. Its increased use in The coontie, an unusual Florida native, is a landscapes has encouraged the presence of the rare cycad—a "living fossil." These primitive plants were a atala butterfly, which uses coontie as a larval host dominant form of plant life during the dinosaur age. plant. Landscapers and homeowners can encourage Other introduced cycads commonly grown in Florida either the plant or the butterfly by following the are: suggestions in this publication. • Cardboard plant/Mexican zamia (Zamia furfuracea) http://hort.ifas.ufl.edu/shrubs/zamfura.pdf • King sago (Cycas revoluta) http://hort.ifas.ufl.edu/shrubs/cycrev.pdf • Queen sago (Cycas rumphii) (Previously called C. circinalis.) http://edis.ifas.ufl.edu/FP161 Coontie Species. There are many different opinions as to the correct name of the species of coontie found growing in Florida. The predominant taxonomic opinion is that there is a single coontie Figure 1. Coonties are a desirable native plant for Florida landscapes. (Photo: Dan Culbert, UF/IFAS) species in Florida (Zamia floridana), while others feel the coontie is represented by several species. Other species names proposed by one or more botanists for the Florida coontie include Z. integrifolia, Z. pumila, and Z. umbrosa. Z. pumila is 1. This document is Fact Sheet ENH 117, a series of the Environmental Horticulture Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. -

Investigations Into Encephalartos Insect Pests and Diseases in South Africa Identifies Phytophthora Cinnamomi As a Pathogen of the Modjadji Cycad
Investigations into Encephalartos insect pests and diseases in South Africa identifies Phytophthora cinnamomi as a pathogen of the Modjadji cycad R. Nesamari1, T.A. Coutinho1 and J. Roux 2* 1Department of Microbiology and Plant Pathology, DST & NRF Centre of Excellence in Tree Health Biotechnology (CTHB), Faculty of Natural and Agricultural Sciences, University of Pretoria, Private Bag X20, Hatfield, Pretoria, 0028 South Africa 2Department of Plant and Soil Sciences, CTHB, FABI, Faculty of Natural and Agricultural Sciences, University of Pretoria, Pretoria, South Africa * Email corresponding author: [email protected] South Africa holds the greatest diversity of Encephalartos species globally. I In recent years several reports have been received of Encephalartos species in the country dying of unknown causes. The aim of this study was to investigate the presence of, and identify the causal agents of diseases of Encephalartos species in the Gauteng and Limpopo Provinces of South Africa. Symptomatic plant material and insects were collected from diseased plants in private gardens, commercial nurseries and conservation areas in these regions. Insects collected were identified based on morphology, and microbial isolates based on morphology and DNA sequence data. Insect species identified infesting cultivated cycads included the beetle Amorphocerus talpa, and the scale insects, Aonidiella aurantii, Aspidiotus capensis, Chrysomphalus aonidum, Lindingaspis rossi, Pseudaulacaspis cockerelli, Pseudaulacaspis pentagona and Pseudococcus longispinus. Fungal species isolated from diseased plants included species of Diaporthe, Epicoccum, Fusarium, Lasiodiplodia, Neofusicoccum, Peyronellaea, Phoma, Pseudocercospora and 1 Toxicocladosporium. The plant pathogen Phytophthora cinnamomi was identified from E. transvenosus plants in the Modjadji Nature Reserve. Artificial inoculation studies fulfilled Koch‟s postulates, strongly suggesting that P. -

Targioni-Tozzetti) (Hemiptera: Pseudococcidae) in Order to Assess Its Importance As a Carrier of Pathogenic Microorganisms T
103 Bulgarian Journal of Agricultural Science, 22 (No 1) 2016, 103-107 Agricultural Academy INVESTIGATION ON THE MICROFLORA OF THE LONG-TAILED MEALYBUG PSEUDOCOCCUS LONGISPINUS (TARGIONI-TOZZETTI) (HEMIPTERA: PSEUDOCOCCIDAE) IN ORDER TO ASSESS ITS IMPORTANCE AS A CARRIER OF PATHOGENIC MICROORGANISMS T. P. POPOVA*, K. G. TRENCHEVA and R. I. TOMOV University of Forestry, BG - 1756 Sofia, Bulgaria Abstract POPOVA, T. P., K. G. TRENCHEVA and R. I. TOMOV, 2016. Investigation on the microflora of the long- tailed mealybug Pseudococcus longispinus (Targioni-Tozzetti) (Hemiptera: Pseudococcidae) in order to assess its importance as a carrier of pathogenic microorganisms. Bulg. J. Agric. Sci., 22: 103–107 Investigations on the microflora of the long-tailed mealybug Pseudococcus longispinus (Targioni-Tozzetti) (Hemiptera: Pseudococcidae) were performed. The following microorganisms were isolated: Salmonella entericа, Serratia plymutica, Enterobacter agglomerans, Staphylococcus cohnii, Bacillus spp., Clostridium spp., Listeria sp., Candida krusei, Penicillium sp., Aspergillus fumigatus. The results from the investigations show that the scale insect could be a reservoir and distributor of conditionally patho- genic for animals and human microorganisms such as bacteria mainly from the family Enterobacteriaceae, staphylococci, spore-forming as well as fungi from the genera Candida and Aspergillus. The presence of fungi of the genus Penicillium is a prerequisite for the development of poliresistance of the identified bac- teria to β-lactams which are among the most widely used antibiotics. Such resistance of the microorganisms isolated from P. longispinus was found in vitro by us in this study through the agar-gel diffusion method of Bauer et al. (1966). The coexistence of bacteria and fungi in insects is proving to be a factor which induces development of resistance of bacteria to antibiotics emit- ted by fungi and probably is one of the reasons for the existence and spread of resistant strains. -

Mealybug Factsheet.Pub
Mealybugs A pest of a different scale Background Mealybugs are a specific type of scale insect from the family Pseudococcidae. They often secrete a thin covering of mealy wax across their body, hence their common name. Like other scale insects, mealybugs are sucking pests that can be present Nursery Production Plant Health & Biosecurity Project & Biosecurity Plant Health Production Nursery across all of Australia on many host plant species. Some species of mealybugs are very serious pests of particular plant species, others are not. Some species may feed on a large number of host plant species, others only on a small number. Mealybugs are most often present on leaves and stems, particularly in tight, protected spaces. However, Nursery levy at work: some mealybugs feed on roots. For information on other types of scale insects, refer to the scale insect factsheet available on the NGIA website. There are a number of mealybug species that may be commonly encountered in production nurseries,;these include longtailed mealybug (Pseudococcus longispinus), obscure mealybug, (P. affinis), citrus mealybug (Planococcus citri), ground mealybugs (Rhizoecus spp.) and many others. Some native mealybug species are common problems on native plants, e.g. Melanococcus albizziae can kill Acacia and Australicoccus grevilleae can cause significant damage to Grevillea. It is likely that at least one mealybug species will feed on most plant species found in a production nursery, at least from time to time. It is beyond the scope of this factsheet to detail the Fig. 1. Adult, egg masses and immature citrus mealybug biology and host range of every mealybug species individuals, photo by the United States National Collection that could be present in production nurseries across of Scale Insects Photographs, bugwood.org. -

Scale Insects Photo Guide
Biodiversity and agriculture: addressing scale insect threats in Kenya Scale insects photo guide August 2020 Authors: Fernadis Makale (CABI), Gillian Watson (NHM), Pamela Kibwage (KEPHIS) and Monica K. Kansiime (CABI) Contributors: Dr. Eston Mutitu (KEFRI), Dr. Muo Kasina (KALRO), Dr. Wanja Kinuthia (NMK), Prof. John Nderitu (UoN), Dr. Andrew Polaszek (NHM), Dr. Mary Mwari Guantai (KEPHIS), Mr. George Opondo (KEFRI) This fact sheet booklet was produced as part of the Darwin Initiative funded project “Biodiversity and agriculture: addressing scale insect threats in Kenya”. The project was jointly implemented by The Natural History Museum UK, National Museums of Kenya (NMK), Kenya Agricultural and Livestock Research Organisation (KALRO), Kenya Forestry Research Institute (KEFRI), University of Nairobi (UoN), Kenya Plant Health Inspectorate Services (KEPHIS), and Centre for Agriculture and Biosciences International (CABI) 1 Introduction What are Scale Insects? Scale insects are a group of sap-sucking insects that insert their tiny, straw like mouthparts into the bark, fruit, or leaves, mostly on trees and shrubs and other perennial plants. Adult females typically have soft bodies and sometimes no limbs and may be concealed underneath domed scales, extruding quantities of wax for protec- tion. The presence of scales can be easily overlooked, in part because they do not resemble most other insects and are easily mistaken for a disease or symptoms. Scale insects are broken into three major distinct groups i.e. Coccidae (soft scales); Diaspididae (armoured scales); and Pseudococcidae (mealybugs) On Kenyan farms, most scales are serious pests of agriculture and forestry. They attack a wide range of plant species including crops of economic importance in Kenya e.g. -

Liabilities Shall Be Attached for Any Mistakes Recorded in This Compiled Version THIS IS an UPDATED and COMPREHENSIVE VERSION, INCLUDES ALL
This compilation is made ONLY for the purpose of easy reference for the general public. No claims/ liabilities shall be attached for any mistakes recorded in this compiled version THIS IS AN UPDATED AND COMPREHENSIVE VERSION, INCLUDES ALL AMENDMENTS TO PLANT QUARANTINE ORDER, 2003 PLANT QUARANTINE (REGULATION OF IMPORT INTO INDIA) ORDER, 2003 S.O.1322 (E). ___ In exercise of the powers conferred by sub-section (1) of Section 3 of the Destructive Insects and Pests Act, 1914 (2 of 1914), the Central Government hereby makes the following Order, for the purpose of prohibiting and regulating the import into India of agricultural articles mentioned herein, namely:- Amendments incorporated as on January, 2008: Plant Quarantine (Regulation of Import into India) Order, 2003: S.O.No.1322 (E), dated 18 th November, 2003 Amendment 1 of 2004 : S.O.No.167(E), dated 6 th February, 2004 Amendment 2 of 2004 : S.O.No.427(E), dated 29 th March, 2004 Amendment 3 of 2004 : S.O.No.644(E), dated 31 st May, 2004 ; Amendment 2 of 2005: S.O. No.263 (E), dated 25 th February, 2005 Amendment 1 of 2005: S.O. No. 462 (E), dated 31 st March, 2005 Plant Quarantine (Regulation of Import India) Amendment Order, 2006: S.O.No.: 1121(E), dated 14 th July, 2006 & S.O. No.1353, dated 31 st July, 2006 2nd Amendment Order, 2006 : S.O.No. 2074(E), dated 6 th December, 2006 3rd Amendment Order, 2006 : S.O.No. 1873(E), dated 31 st October, 2006 1st Amendment Order, 2007 : S.O.