Consolidated Version of the Sanpin 2.3.2.1078-01 on Food, Raw Material, and Foodstuff
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Registered with the Ministry of Justice of the RF, March 22, 2002 No. 3326
Registered with the Ministry of Justice of the RF, March 22, 2002 No. 3326 MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION CHIEF STATE SANITARY INSPECTOR OF THE RUSSIAN FEDERATION RESOLUTION No. 36 November 14, 2001 ON ENACTMENT OF SANITARY RULES (as amended by Amendments No.1, approved by Resolution No. 27 of Chief State Sanitary Inspector of the RF dated 20.08.2002, Amendments and Additions No. 2, approved by Resolution No. 41 of Chief State Sanitary Inspector of the RF dated15.04.2003, No. 5, approved by Resolution No. 42 of Chief State Sanitary Inspector of the RF dated 25.06.2007, No. 6, approved by Resolution No. 13 of Chief State Sanitary Inspector of the RF dated 18.02.2008, No. 7, approved by Resolution No. 17 of Chief State Sanitary Inspector of the RF dated 05.03.2008, No. 8, approved by Resolution No. 26 of Chief State Sanitary Inspector of the RF dated 21.04.2008, No. 9, approved by Resolution No. 30 of Chief State Sanitary Inspector of the RF dated 23.05.2008, No. 10, approved by Resolution No. 43 of Chief State Sanitary Inspector of the RF dated 16.07.2008, Amendments No.11, approved by Resolution No. 56 of Chief State Sanitary Inspector of the RF dated 01.10.2008, No. 12, approved by Resolution No. 58 of Chief State Sanitary Inspector of the RF dated 10.10.2008, Amendment No. 13, approved by Resolution No. 69 of Chief State Sanitary Inspector of the RF dated 11.12.2008, Amendments No.14, approved by Resolution No. -
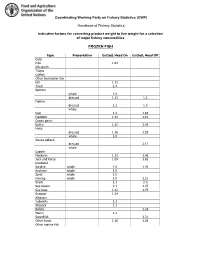
Indicative Factors for Converting Product Weight to Live Weight for a Selection of Major Fishery Commodities
Coordinating Working Party on Fishery Statistics (CWP) Handbook of Fishery Statistics Indicative factors for converting product weight to live weight for a selection of major fishery commodities FROZEN FISH Type Prese tatio Gutted, Head O Gutted, Head Off Carp Pike 1.04 Nile perch Tilapia Catfish Other freshwater fish Eel 1.11 Trout 1.4 Salmon: whole 1.0 dressed 1.1 1. Flatfish: dressed 1.1 1.4 whole Cod 1.2 1.69 Haddock 1.14 1.61 Ocean perch Saithe 1.15 1.45 Hake: dressed 1.16 1.55 whole 1.0 Alaska pollack: dressed 2.17 whole Capelin Mackerel 1.11 1.46 Jack and horse 1.09 1.65 mackerel Sardine whole 1.0 1.4 Anchovy whole 1.0 Sprat whole 1.0 Herring whole 1.0 1.52 Shark 1.1 2.0 Sea .ream 1.1 1.47 Sea .ass 1.12 1.79 Snapper 1.14 Al.acore /ellowfin 1.1 Skip0ack 1.1 1onito 1.29 Marlin 1.1 Swordfish 1. 1 Other tunas 1.16 1. 6 Other marine fish FILLETS Type Prese tatio Raw CF Ski Off CF Flatfish 2.42 2.61 Herring 1.62 2.17 Cod 2.45 .2 fillet .2 portion .55 mince .2 Mackerel 1.95 2.60 Haddock 2.77 2.91 fillet 2.21 portion .20 Ocean perch 2.92 .11 Saithe 2.12 2.55 Hake 2.5 2.90 fillet 2.90 portion .19 mince 2.90 Alaska pollack .72 fillet .72 mince .72 Salmon 2.00 2.00 steaks 1.60 Tuna 1.54 1.92 loins Catfish 2.6 .55 steaks 2.40 FISH DRIED, WHETHER OR NOT SALTED Basic Conversion Factor for Basic Type Deduced CF Product Product Stockfish gutted cod 1.2 1.94 Klipfish filleted cod 2.45 3.97 Hake fillet 2.53 4.1 Shark fillet 2.4 3.89 Shark fins 0 Tuna fillet 1.54 2.49 Pilchard fillet 1.62 2.62 Tilapia gutted 1.2 1.94 Other freshwater fillet 2.45 4 Other fish fillet 2.45 4 N1: the conversion factor for dried fish is taken as 1.62 times the CF for the .asic product FISH SALTED, WET OR IN BRINE Type O,served Co versio Fa.tors Mea or 3range4 Re.omme ded CF Freshwater fish 1.5 Cod 1.79 5 2.2 2.0 Other demersal 1. -
Structures Assisting the Migrations of Non-Salmonid Fish: USSR
FAO Structures assisting FISHERIES TECHNICAL the migrations of PAPER non-salmonid fish: USSR 308 D.S. Pavlov Severtsov's Institute of the Evolutionary Morphology and Ecology of Animals Leninskii Prospekt 33 117071 Moscow B-71, USSR FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 1989 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-42 ISBN 92-5-102857-5 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechani- cal, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations, Via delle Terme di Caracalla, 00100 Rome, Italy. 0 FAO 1989 PREPARATION OF THIS DOC UMENT The Working Party of Experts on Inland Fisheries of the Indo-Pacific Commission, at a meeting in New Delhi, India, in January 1984, recommended that information be collected on the use of fish-passes. s a result of this recommenda- tion, FAO commissioned a consultant to review stn ctures assisting migration of non-salmonid stocks in the USSR. The original manuscript has been subject to substantial editing by Drs R. -

Szynka Drobiowa
Zakłady Mięsne Silesia S.A. w swoim portfolio posiadają szeroką ofertę produktową dostępną pod pięcioma markami handlowymi. Każda z marek dedykowana jest innej kategorii produktowej. Marka nowoczesna, przyjazna i rodzinna. Produkty na bazie mięsa wieprzowego i drobiowego o bardzo dobrej relacji ceny do jakości. Marka znana od 30 lat, posiadająca grono lojalnych konsumentów. mięso drobiowe, mięso wieprzowe, klasyki wśród produktów Marka dedykowana produktom premium. Produkty w tej linii tworzone są w oparciu o starannie wyselekcjonowane składniki oraz sprawdzone receptury. Duda Specialite to idealne rozwiązanie dla tych, którzy poszukują unikalnych smaków i cenią wysoką jakość produktów. marka produktów najwyższej jakości To wędliny drobiowe z mięsa kurczaka, kury i gęsi. Odpowiednio doprawione stanowią smaczną alternatywę dla wędlin wieprzowych. wędliny drobiowe, kurczak, kura, gęś Dania gotowe z mięsa drobiowego z dodatkiem warzyw, sera, aromatycznych przypraw, panierowane bądź nie. Gotowe do spożycia na zimno lub po podgrzaniu w piekarniku, na patelni lub mikrofalówce. dania gotowe, mięso drobiowe Produkty stworzone z myślą o czworonogach. Doskonale zbilansowa- ne karmy dla psów oraz kotów to źródło cennych witamin i mikroele- mentów niezbędnych dla ich prawidłowego rozwoju. Argos to również naturalne gryzaki drobiowe, wieprzowe i wołowe, które stanowią znakomite uzupełnienie codziennej diety pupila. karmy i przysmaki dla psów i kotów wędliny 69 g mięsa wędliny PREMIUM DLA DZIECI wieprzowego 30 g mięsa z jelenia DUDA SPECIALITE 9 g -

View Travel Planning Guide
YOUR O.A.T. ADVENTURE TRAVEL PLANNING GUIDE® New! Under the Midnight Sun: Sami Lapland, Norway & the Arctic Circle 2021 Small Groups: 8-16 travelers—guaranteed! (average of 13) Overseas Adventure Travel ® The Leader in Personalized Small Group Adventures on the Road Less Traveled 1 Dear Traveler, At last, the world is opening up again for curious travel lovers like you and me. And the O.A.T. New! Under the Midnight Sun: Sami Lapland, Norway & the Arctic Circle itinerary you’ve expressed interest in will be a wonderful way to resume the discoveries that bring us so much joy. You might soon be enjoying standout moments like these: There was something intangibly magical about Lapland. Maybe it was the midnight sun, the endless rugged tundra, or the welcoming nature of the Sami people. All I know is that there was a true sense of Arctic magic everywhere I went, especially when I met an indigenous Sami family on their reindeer farm. As we explored the farm, they introduced me to their way of life and traditions dating back thousands of years. I was saddened to hear that their ancient culture is under threat from two forces: the construction of an Arctic Railway through Sami territory and Sami youth deviating from their traditional lifestyle. You’ll hear about these challenges as well when you meet with a Sami family on their reindeer farm. In the regions I travel to around the world, the stories of the people who live and work there are the most distinct and poignant experiences. You’ll meet with a local educator in Oslo to hear about July 22, 2011—the harrowing terrorist attack on this city—and their personal account of this day. -

UCSC Special Collections and Archives MS 6 Morley Baer
UCSC Special Collections and Archives MS 6 Morley Baer Photographs - Job Number Index Description Job Number Date Thompson Lawn 1350 1946 August Peter Thatcher 1467 undated Villa Moderne, Taylor and Vial - Carmel 1645-1951 1948 Telephone Building 1843 1949 Abrego House 1866 undated Abrasive Tools - Bob Gilmore 2014, 2015 1950 Inn at Del Monte, J.C. Warnecke. Mark Thomas 2579 1955 Adachi Florists 2834 1957 Becks - interiors 2874 1961 Nicholas Ten Broek 2878 1961 Portraits 1573 circa 1945-1960 Portraits 1517 circa 1945-1960 Portraits 1573 circa 1945-1960 Portraits 1581 circa 1945-1960 Portraits 1873 circa 1945-1960 Portraits unnumbered circa 1945-1960 [Naval Radio Training School, Monterey] unnumbered circa 1945-1950 [Men in Hardhats - Sign reads, "Hitler Asked for It! Free Labor is Building the Reply"] unnumbered circa 1945-1950 CZ [Crown Zellerbach] Building - Sonoma 81510 1959 May C.Z. - SOM 81552 1959 September C.Z. - SOM 81561 1959 September Crown Zellerbach Bldg. 81680 1960 California and Chicago: landscapes and urban scenes unnumbered circa 1945-1960 Spain 85343 1957-1958 Fleurville, France 85344 1957 Berardi fountain & water clock, Rome 85347 1980 Conciliazione fountain, Rome 84154 1980 Ferraioli fountain, Rome 84158 1980 La Galea fountain, in Vatican, Rome 84160 1980 Leone de Vaticano fountain (RR station), Rome 84163 1980 Mascherone in Vaticano fountain, Rome 84167 1980 Pantheon fountain, Rome 84179 1980 1 UCSC Special Collections and Archives MS 6 Morley Baer Photographs - Job Number Index Quatre Fountain, Rome 84186 1980 Torlonai -

WO 2013/116261 A2 8 August 2013 (08.08.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/116261 A2 8 August 2013 (08.08.2013) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every CUD 3/386 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US2013/023728 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 30 January 2013 (30.01 .2013) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (26) Publication Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (30) Priority Data: ZM, ZW. 12000745.5 3 February 2012 (03.02.2012) EP 12001034.3 16 February 2012 (16.02.2012) EP (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant: THE PROCTER & GAMBLE COMPANY GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, [US/US]; One Procter & Gamble Plaza, Cincinnati, Ohio UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, 45202 (US). -

A Review of Guidance on Fish Consumption in Pregnancy: Is It Fit for Purpose? Public Health Nutrition
Taylor, C. , Emmett, P., Emond, A., & Golding, J. (2018). A review of guidance on fish consumption in pregnancy: Is it fit for purpose? Public Health Nutrition. https://doi.org/10.1017/S1368980018000599 Publisher's PDF, also known as Version of record License (if available): CC BY Link to published version (if available): 10.1017/S1368980018000599 Link to publication record in Explore Bristol Research PDF-document This is the final published version of the article (version of record). It first appeared online via Cambridge University Press at https://www.cambridge.org/core/journals/public-health-nutrition/article/review-of-guidance-on- fish-consumption-in-pregnancy-is-it-fit-for-purpose/BC3BB20A2D848F5CF5AED90C86413F85 . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ Public Health Nutrition: page 1 of 11 doi:10.1017/S1368980018000599 Review Article A review of guidance on fish consumption in pregnancy: is it fit for purpose? Caroline M Taylor*, Pauline M Emmett, Alan M Emond and Jean Golding Centre for Child and Adolescent Health, Population Health Sciences, Bristol Medical School, University of Bristol, Oakfield House, Oakfield Grove, Bristol BS8 2BN, UK Submitted 17 November 2017: Final revision received 14 February 2018: Accepted 14 February 2018 Abstract Objective: Public health messages to reduce Hg exposure for pregnant women have focused exclusively on advice on fish consumption to limit Hg exposure, with little account being taken of the positive contribution of fish to nutritional quality. -

Super Greens
SUPER GREENS SUPPLEMENT FACTS Serving Size: 1 Scoop (10 g) Servings Per Container: 30 Amount Per Serving % Daily Value Calories 25 Total Carbohydrate 6 g 2%* Dietary Fiber 3 g 12%* Sugars 1 g † Protein <1 g <2%* Organic Super Greens, Sprouts & Prebiotic Fiber Blend: 3.5 g Wheat grass, barley grass, spirulina (blue green algae), green pea fiber, oat fiber, flaxseed, blue agave inulin, plum, orange peel, apple fiber, lemon peel, kale, broccoli, spinach, parsley, green cabbage, alfalfa grass, chlorella, green tea, tomato, sweet potato, pumpkin, dandelion root, collard greens, dulse, adzuki sprout, amaranth sprout, buckwheat sprout, chia sprout, flax sprout, garbanzo bean sprout, lentil bean sprout, millet sprout, pumpkin sprout, quinoa sprout, sesame sprout, sunflower sprout Organic Super Foods & Fruits Blend: 3.5 g Beet root, carrot, Organic Berry Blend [acai berry (Euterpe oleracea), apple, banana, bilberry, black currant, blueberry, cherry, elderberry, goji berry (Lycium barbarum), grape, maqui berry (Aristotelia chilensis), papaya, pineapple, pomegranate, raspberry, strawberry], apple, shitake mushroom, reishi mushroom, maitake mushroom, blueberry, raspberry, strawberry, peach, blackberry, lemon, pear, cranberry, grape, pomegranate, cherry, orange Probiotic Blend: 500 million CFU Bacillus coagulans, Lactobacillus rhamnosus, Bifidobacterium bifidum, Bifidobacterium longum, Lactobacillus acidophilus, Lactobacillus casei, Streptococcus thermophilus * Percent Daily Values are based on a 2,000 calorie diet. † Daily value not established. Other ingredients: Organic tapioca starch, organic guar gum, citric acid, organic natural berry flavor and organic rebaudioside A. . -

AFS – Advances in Food Sciences Continuation of CMTL Founded by F
AFS – Advances in Food Sciences Continuation of CMTL founded by F. Drawert Production by PSP – Parlar Scientific Publications, Angerstr. 12, 85354 Freising, Germany in cooperation with Lehrstuhl für Chemisch-Technische Analyse und Lebensmitteltechnologie, Technische Universität München, 85350 Freising - Weihenstephan, Germany Copyright © by PSP – Parlar Scientific Publications, Angerstr. 12, 85354 Freising, Germany. All rights are reserved, especially the right to translate into foreign language. No part of the journal may be reproduced in any form- through photocopying, microfilming or other processes- or converted to a machine language, especially for data processing equipment- without the written permission of the publisher. The rights of reproduction by lecture, radio and television transmission, magnetic sound recording or similar means are also reserved. Printed in GERMANY – ISSN 14311431----77377737 © by PSP Volume 24 – No 4. 2002 Advances in Food Sciences 1 © by PSP Volume 24 – No 4. 2002 Advances in Food Sciences AFSAFS---- Editorial Board Chief Editors: Prof. Dr. H. Parlar Institut für Lebensmitteltechnologie und Analytische Chemie, TU München - 85350 Freising-Weihenstephan, Germany - E-mail: [email protected] Dr. G. Leupold Institut für Lebensmitteltechnologie und Analytische Chemie, TU München - 85350 Freising-Weihenstephan, Germany - E-mail: [email protected] CoCo----Editor:Editor: Prof. Dr. R. G. Berger Zentrum Angewandte Chemie, Institut für Lebensmittelchemie, Universität Hannover Wunstorfer Straße 14, 30453 Hannover - E-mail: [email protected] AFSAFS- Advisory Board E. Anklam, I M. Bahadir, D F. Coulston, USA J.M. de Man, CAN N. Fischer, D S. Gäb, D A. Görg, D U. Gill, CAN D. Hainzl, P W.P. Hammes, D D. Kotzias, I F. -

Medicine Plants of Folk Medicine Used for Treatment of Gastro-Intestinal Problems in Fergana Valley
국내․외 기술정보 Medicine plants of folk medicine used for treatment of gastro-intestinal problems in Fergana valley Valeriy V. Pak 식품기능연구본부 This article presents a review of indigenous medicinal plants used in folk medicine in Fergana valley (Uzbekistan) for treatment of gastro-intestinal problems. The 29 different plantsbelong to 18 different plant spices are presented. The methods of preparation of remedies and utilized parts of plants are described. Ⅰ. Introduction The purpose of this article is to review the remedies of the folk medicine for treatment of Plant products – as part of foods or botanical gastro-intestinal problems used in Fergana portions and powder – have been used with valley presenting the most densely populated varying success to cure and prevent diseases part of Uzbekistan. throughout history. Several diverse line of evidence indicates that medicinal plants represent the oldest and most widespread form of Ⅱ. Geographic characteristic medication. Until recently, plants were an of Fergana valley important source for the discovery of novel pharmacologically active compounds, with many Fergana valley occupiesa territory about 22.000 blockbuster drugs being derived directly or sq km and divided among Uzbekistan, Tajikistan indirectly from plants [1,2]. As it is estimated and Kyrgystan (Fig. 1). The Fergana Range by World Health Organization (WHO) that 25 % rises in the northeast and the Pamir in the of the active compounds in currently prescribed south. The Gissar and Alay ranges stand across synthetic drugs were first identified in plant the Fergana valley, which lies south of the sources [3]. Thus, to collect information about western Tian-Shan. The Xinjiang region of medicine plant used in folk medicine is valuable China borders the valley in the southeast. -

A Molecular Phylogeny of the Solanaceae
TAXON 57 (4) • November 2008: 1159–1181 Olmstead & al. • Molecular phylogeny of Solanaceae MOLECULAR PHYLOGENETICS A molecular phylogeny of the Solanaceae Richard G. Olmstead1*, Lynn Bohs2, Hala Abdel Migid1,3, Eugenio Santiago-Valentin1,4, Vicente F. Garcia1,5 & Sarah M. Collier1,6 1 Department of Biology, University of Washington, Seattle, Washington 98195, U.S.A. *olmstead@ u.washington.edu (author for correspondence) 2 Department of Biology, University of Utah, Salt Lake City, Utah 84112, U.S.A. 3 Present address: Botany Department, Faculty of Science, Mansoura University, Mansoura, Egypt 4 Present address: Jardin Botanico de Puerto Rico, Universidad de Puerto Rico, Apartado Postal 364984, San Juan 00936, Puerto Rico 5 Present address: Department of Integrative Biology, 3060 Valley Life Sciences Building, University of California, Berkeley, California 94720, U.S.A. 6 Present address: Department of Plant Breeding and Genetics, Cornell University, Ithaca, New York 14853, U.S.A. A phylogeny of Solanaceae is presented based on the chloroplast DNA regions ndhF and trnLF. With 89 genera and 190 species included, this represents a nearly comprehensive genus-level sampling and provides a framework phylogeny for the entire family that helps integrate many previously-published phylogenetic studies within So- lanaceae. The four genera comprising the family Goetzeaceae and the monotypic families Duckeodendraceae, Nolanaceae, and Sclerophylaceae, often recognized in traditional classifications, are shown to be included in Solanaceae. The current results corroborate previous studies that identify a monophyletic subfamily Solanoideae and the more inclusive “x = 12” clade, which includes Nicotiana and the Australian tribe Anthocercideae. These results also provide greater resolution among lineages within Solanoideae, confirming Jaltomata as sister to Solanum and identifying a clade comprised primarily of tribes Capsiceae (Capsicum and Lycianthes) and Physaleae.