HIGHLIGHTS of PRESCRIBING INFORMATION These Highlights Do
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amerithrax Investigative Summary
The United States Department of Justice AMERITHRAX INVESTIGATIVE SUMMARY Released Pursuant to the Freedom of Information Act Friday, February 19, 2010 TABLE OF CONTENTS I. THE ANTHRAX LETTER ATTACKS . .1 II. EXECUTIVE SUMMARY . 4 A. Overview of the Amerithrax Investigation . .4 B. The Elimination of Dr. Steven J. Hatfill as a Suspect . .6 C. Summary of the Investigation of Dr. Bruce E. Ivins . 6 D. Summary of Evidence from the Investigation Implicating Dr. Ivins . .8 III. THE AMERITHRAX INVESTIGATION . 11 A. Introduction . .11 B. The Investigation Prior to the Scientific Conclusions in 2007 . 12 1. Early investigation of the letters and envelopes . .12 2. Preliminary scientific testing of the Bacillus anthracis spore powder . .13 3. Early scientific findings and conclusions . .14 4. Continuing investigative efforts . 16 5. Assessing individual suspects . .17 6. Dr. Steven J. Hatfill . .19 7. Simultaneous investigative initiatives . .21 C. The Genetic Analysis . .23 IV. THE EVIDENCE AGAINST DR. BRUCE E. IVINS . 25 A. Introduction . .25 B. Background of Dr. Ivins . .25 C. Opportunity, Access and Ability . 26 1. The creation of RMR-1029 – Dr. Ivins’s flask . .26 2. RMR-1029 is the source of the murder weapon . 28 3. Dr. Ivins’s suspicious lab hours just before each mailing . .29 4. Others with access to RMR-1029 have been ruled out . .33 5. Dr. Ivins’s considerable skill and familiarity with the necessary equipment . 36 D. Motive . .38 1. Dr. Ivins’s life’s work appeared destined for failure, absent an unexpected event . .39 2. Dr. Ivins was being subjected to increasing public criticism for his work . -

Investigation of Anthrax Associated with Intentional Exposure
October 19, 2001 / Vol. 50 / No. 41 889 Update: Investigation of Anthrax Associated with Intentional Exposure and Interim Public Health Guidelines, October 2001 893 Recognition of Illness Associated with the Intentional Release of a Biologic Agent 897 Weekly Update: West Nile Virus Activity — United States, October 10–16, 2001 Update: Investigation of Anthrax Associated with Intentional Exposure and Interim Public Health Guidelines, October 2001 On October 4, 2001, CDC and state and local public health authorities reported a case of inhalational anthrax in Florida (1 ). Additional cases of anthrax subsequently have been reported from Florida and New York City. This report updates the findings of these case investigations, which indicate that infections were caused by the intentional release of Bacillus anthracis. This report also includes interim guidelines for postexposure pro- phylaxis for prevention of inhalational anthrax and other information to assist epidemi- ologists, clinicians, and laboratorians responding to intentional anthrax exposures. For these investigations, a confirmed case of anthrax was defined as 1) a clinically compatible case of cutaneous, inhalational, or gastrointestinal illness* that is laboratory confirmed by isolation of B. anthracis from an affected tissue or site or 2) other labora- tory evidence of B. anthracis infection based on at least two supportive laboratory tests. A suspected case was defined as 1) a clinically compatible case of illness without isola- tion of B. anthracis and no alternative diagnosis, but with laboratory evidence of B. anthracis by one supportive laboratory test or 2) a clinically compatible case of an- thrax epidemiologically linked to a confirmed environmental exposure, but without cor- roborative laboratory evidence of B. -

Comparative Genome Analysis of 19 Ureaplasma Urealyticum and Ureaplasma Parvum Strains
Paralanov et al. BMC Microbiology 2012, 12:88 http://www.biomedcentral.com/1471-2180/12/88 RESEARCH ARTICLE Open Access Comparative genome analysis of 19 Ureaplasma urealyticum and Ureaplasma parvum strains Vanya Paralanov1, Jin Lu2, Lynn B Duffy2, Donna M Crabb2, Susmita Shrivastava1, Barbara A Methé1, Jason Inman1, Shibu Yooseph1, Li Xiao2, Gail H Cassell2, Ken B Waites2 and John I Glass1* Abstract Background: Ureaplasma urealyticum (UUR) and Ureaplasma parvum (UPA) are sexually transmitted bacteria among humans implicated in a variety of disease states including but not limited to: nongonococcal urethritis, infertility, adverse pregnancy outcomes, chorioamnionitis, and bronchopulmonary dysplasia in neonates. There are 10 distinct serotypes of UUR and 4 of UPA. Efforts to determine whether difference in pathogenic potential exists at the ureaplasma serovar level have been hampered by limitations of antibody-based typing methods, multiple cross-reactions and poor discriminating capacity in clinical samples containing two or more serovars. Results: We determined the genome sequences of the American Type Culture Collection (ATCC) type strains of all UUR and UPA serovars as well as four clinical isolates of UUR for which we were not able to determine serovar designation. UPA serovars had 0.75−0.78 Mbp genomes and UUR serovars were 0.84−0.95 Mbp. The original classification of ureaplasma isolates into distinct serovars was largely based on differences in the major ureaplasma surface antigen called the multiple banded antigen (MBA) and reactions of human and animal sera to the organisms. Whole genome analysis of the 14 serovars and the 4 clinical isolates showed the mba gene was part of a large superfamily, which is a phase variable gene system, and that some serovars have identical sets of mba genes. -

2001 Anthrax Letters
Chapter Five: 2001 Anthrax Letters Author’s Note: The analysis and comments regarding the communication efforts described in this case study are solely those of the authors; this analysis does not represent the official position of the FDA. This case was selected because it is one of the few major federal efforts to distribute medical countermeasures in response to an acute biological incident. These events occurred more than a decade ago and represent the early stages of US biosecurity preparedness and response; however, this incident serves as an excellent illustration of the types of communication challenges expected in these scenarios. Due in part to the extended time since these events and the limited accessibility of individual communications and messages, this case study does not provide a comprehensive assessment of all communication efforts. In contrast to the previous case studies in this casebook, the FDA’s role in the 2001 anthrax response was relatively small, and as such, this analysis focuses principally on the communication efforts of the CDC and state and local public health agencies. The 2001 anthrax attacks have been studied extensively, and the myriad of internal and external assessments led to numerous changes to response and communications policies and protocols. The authors intend to use this case study as a means of highlighting communication challenges strictly within the context of this incident, not to evaluate the success or merit of changes made as a result of these events. Abstract The dissemination of Bacillus anthracis via the US Postal Service (USPS) in 2001 represented a new public health threat, the first intentional exposure to anthrax in the United States. -

Roles of Oral Bacteria in Cardiovascular Diseases—From
J Pharmacol Sci 113, 000 – 000 (2010) Journal of Pharmacological Sciences ©2010 The Japanese Pharmacological Society Forum Minireview Roles of Oral Bacteria in Cardiovascular Diseases — From Molecular Mechanisms to Clinical Cases: Implication of Periodontal Diseases in Development of Systemic Diseases Hiroaki Inaba1 and Atsuo Amano1,* 1Department of Oral Frontier Biology, Graduate School of Dentistry, Osaka University, Suita 565-0871, Japan Received November 16, 2009; Accepted December 21, 2009 Abstract. Periodontal diseases, some of the most common infectious diseases seen in humans, are characterized by gingival inflammation, as well as loss of connective tissue and bone from around the roots of the teeth, which leads to eventual tooth exfoliation. In the past decade, the as- sociation of periodontal diseases with the development of systemic diseases has received increas- ing attention. Although a number of studies have presented evidence of close relationships between periodontal and systemic diseases, the majority of findings are limited to epidemiological studies, while the etiological details remain unclear. Nevertheless, a variety of recent hypothesis driven investigations have compiled various results showing that periodontal infection and subsequent direct oral-hematogenous spread of bacteria are implicated inPROOF the development of various systemic diseases. Herein, we present current understanding in regard to the relationship between periodon- tal and systemic diseases, including cardiovascular diseases, preterm delivery of low birth weight, diabetes mellitus, respiratory diseases, and osteoporosis. Keywords: periodontal disease, diabetes mellitus, cardiovascular disease, preterm delivery, Porphyromonas gingivalis, oral bacteria 1. Introduction detected in heart valve lesions and atheromatous plaque (5, 6), amniotic fluid of pregnant women with threatened Epidemiological and interventional studies of humans premature labor (11), and placentas from cases of preterm have revealed close associations between periodontal delivery (12, 13). -

Lessons from the Anthrax Attacks Implications for US
Lessons from the Anthrax Attacks Implications for US. Bioterrorism Preparedness A Report on a National Forum on Biodefense Author David Heymart Research Assistants Srusha Ac h terb erg, L Joelle Laszld Organized by the Center for Strategic and International Studies and the Defense Threat Reduction Agency --CFr”V? --.....a DlSTRf BUTION This report ISfor official use only; distribution authorized to U S. government agencies, designated contractors, and those with an official need Contains information that may be exempt from public release under the Freedom of Information Act. exemption number 2 (5 USC 552); exemption number 3 (’lo USC 130).Approvalof the Defense Threat Reduction Agency prior to public release is requrred Contract Number OTRAM-02-C-0013 For Official Use Only About CSIS For four decades, the Center for Stravgic and Internahonal Studies (CSIS)has been dedicated 10 providing world leaders with strategic insights on-and pohcy solubons tcurrentand emergtng global lssues CSIS IS led by John J Hamre, former L S deputy secretary of defense It is guided by a board of trustees chaired by former U S senator Sam Nunn and consistlng of prominent individuals horn both the public and private sectors The CSIS staff of 190 researchers and support staff focus pnrnardy on three subjecr areas First, CSIS addresses the fuU spectrum of new challenges to national and mternabonal security Second, it maintains resident experts on all of the world's myor geographical regions Third, it IS committed to helping to develop new methods of governance for the global age, to this end, CSIS has programs on technology and pubhc policy, International trade and finance, and energy Headquartered in Washington, D-C ,CSIS IS pnvate, bipartlsan, and tax-exempt CSIS docs not take specific policy positions, accordygly, all views expressed herein should be understood to be solely those ofthe author Spousor. -
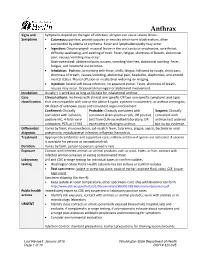
Anthrax Reporting and Investigation Guideline
Anthrax Signs and Symptoms depend on the type of infection; all types can cause severe illness: Symptoms • Cutaneous: painless, pruritic papules or vesicles which form black eschars, often surrounded by edema or erythema. Fever and lymphadenopathy may occur. • Ingestion: Oropharyngeal: mucosal lesion in the oral cavity or oropharynx, sore throat, difficulty swallowing, and swelling of neck. Fever, fatigue, shortness of breath, abdominal pain, nausea/vomiting may occur. Gastrointestinal: abdominal pain, nausea, vomiting/diarrhea, abdominal swelling. Fever, fatigue, and headache are common. • Inhalation: Biphasic, presenting with fever, chills, fatigue, followed by cough, chest pain, shortness of breath, nausea/vomiting, abdominal pain, headache, diaphoresis, and altered mental status. Pleural effusion or mediastinal widening on imaging. • Injection: Severe soft tissue infection; no apparent eschar. Fever, shortness of breath, nausea may occur. Occasional meningeal or abdominal involvement. Incubation Usually < 1 week but as long as 60 days for inhalational anthrax Case Clinical criteria: An illness with at least one specific OR two non-specific symptoms and signs classification that are compatible with one of the above 4 types, systemic involvement, or anthrax meningitis; OR death of unknown cause and consistent organ involvement Confirmed: Clinically Probable: Clinically consistent with Suspect: Clinically consistent with isolation, consistent Gram-positive rods, OR positive consistent with positive IHC, 4-fold rise in test from CLIA-accredited laboratory, OR anthrax test ordered antibodies, PCR, or LF MS epi evidence relating to anthrax but no epi evidence Differential Varies by form; mononucleosis, cat-scratch fever, tularemia, plague, sepsis, bacterial or viral diagnosis pneumonia, mycobacterial infection, influenza, hantavirus Treatment Appropriate antibiotics and supportive care; anthrax antitoxin if spores are activated. -

Bioterrorism, Biological Weapons and Anthrax
Bioterrorism, Biological Weapons and Anthrax Part IV Written by Arthur H. Garrison Criminal Justice Planning Coordinator Delaware Criminal Justice Council Bioterrorism and biological weapons The use of bio-terrorism and bio-warfare dates back to 6th century when the Assyrians poisoned the well water of their enemies. The goal of using biological weapons is to cause massive sickness or death in the intended target. Bioterrorism and biological weapons The U.S. took the threat of biological weapons attack seriously after Gulf War. Anthrax vaccinations of U.S. troops Investigating Iraq and its biological weapons capacity The Soviet Union manufactured various types of biological weapons during the 1980’s • To be used after a nuclear exchange • Manufacturing new biological weapons – Gene engineering – creating new types of viruses/bacteria • Contagious viruses – Ebola, Marburg (Filoviruses) - Hemorrhagic fever diseases (vascular system dissolves) – Smallpox The spread of biological weapons after the fall of the Soviet Union •Material • Knowledge and expertise •Equipment Bioterrorism and biological weapons There are two basic categories of biological warfare agents. Microorganisms • living organic germs, such as anthrax (bacillus anthrax). –Bacteria –Viruses Toxins • By-products of living organisms (natural poisons) such as botulism (botulinum toxin) which is a by- product of growing the microorganism clostridium botulinum Bioterrorism and biological weapons The U.S. was a leader in the early research on biological weapons Research on making -

Antibacterial Photosensitization Through Activation of PNAS PLUS Coproporphyrinogen Oxidase
Antibacterial photosensitization through activation of PNAS PLUS coproporphyrinogen oxidase Matthew C. Surdela, Dennis J. Horvath Jr.a, Lisa J. Lojeka, Audra R. Fullena, Jocelyn Simpsona, Brendan F. Dutterb,c, Kenneth J. Sallenga, Jeremy B. Fordd, J. Logan Jenkinsd, Raju Nagarajane, Pedro L. Teixeiraf, Matthew Albertollec,g, Ivelin S. Georgieva,e,h, E. Duco Jansend, Gary A. Sulikowskib,c, D. Borden Lacya,d, Harry A. Daileyi,j,k, and Eric P. Skaara,1 aDepartment of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, Nashville, TN 37232; bDepartment of Chemistry, Vanderbilt University, Nashville, TN 37232; cVanderbilt Institute for Chemical Biology, Nashville, TN 37232; dDepartment of Biomedical Engineering, Vanderbilt University, Nashville, TN 37232; eVanderbilt Vaccine Center, Vanderbilt University Medical Center, Nashville, TN 37232; fBiomedical Informatics, Vanderbilt University School of Medicine, Nashville, TN 37203; gDepartment of Biochemistry, Vanderbilt University, Nashville, TN 37232; hDepartment of Electrical Engineering and Computer Science, Vanderbilt University, Nashville, TN 37232; iBiomedical and Health Sciences Institute, University of Georgia, Athens, GA 30602; jDepartment of Microbiology, University of Georgia, Athens, GA 30602; and kDepartment of Biochemistry and Molecular Biology, University of Georgia, Athens, GA 30602 Edited by Ferric C. Fang, University of Washington School of Medicine, Seattle, WA, and accepted by Editorial Board Member Carl F. Nathan June 26, 2017 (received for review January 10, 2017) Gram-positive bacteria cause the majority of skin and soft tissue Small-molecule VU0038882 (‘882) was previously identified in infections (SSTIs), resulting in the most common reason for clinic a screen for activators of the S. aureus heme-sensing system two- visits in the United States. -

Fis-His-2020-Abstract-Supplement-Free-Paper.Pdf
Abstract supplement (free paper abstracts) FIS/HIS International 2020 cannot be held responsible for errors or inconsistencies contained within the abstract supplement. Only major formatting alterations have been made and abstract content remains consistent with what was entered at time of submission by the author/s. How to search this document Laptop, PC or similar: ‘Ctrl’ + ‘F’ or ‘Edit’ then ‘Find’ Smart device: Open this PDF in either the 'iBooks' app (Apple) or 'My Files' app (Android). Both are built in apps and have a magnifying glass search icon. Contents A quick reference guide by topic, paper number and presentation type can be found at the end of the document or click here. (Click paper title to take you straight there) Free paper oral presentations 11: Investigations, actions and learning from an outbreak of SARS-CoV-2 infection among health care workers in the United Kingdom ........................................................................................................................ 13 12: Procalcitonin as an antibiotic stewardship tool in COVID-19 patients in the intensive care ...................... 14 20: Impact of antibiotic timing on mortality from Gram negative Bacteraemia in an English District General Hospital: the importance of getting it right every time .................................................................................... 15 35: Case report: A fall in the forest ................................................................................................................... 16 65: Not -

CASEE-2017 Session1b -Eva-Tvrda
A The 8th International CASEE Conference Warsaw University of Life Sciences – SGGW May 14 - 16, 2017 . Decreased sperm quality visible in routine semen analysis: . loss of sperm motility . morphological alterations . acrosome dysfunction . disruption of membrane integrity . oxidative stress . Most data connected to bacterial contamination of ejaculates: well-known causative agents of urogenital tract infections . Escherichia coli, Staphylococcus aureus, Ureaplasma urealyticum, Mycoplasma hominis, Chlamydia trachomatis . Ejaculates collected for reproductive technologies - certain contamination: . semen collection is not an entirely serile process . factors for semen contamination: artifical vaginas, environmental conditions, human factors . Current interest shifts to other bacteria, responsible for the colonization and contamination of the male urogenital tract, rather than infection . Gram-positive, catalase-negative, non-spore-forming, facultative anaerobic bacteria . Lactic acid bacteria (LAB) that produce bacteriocins . Origins: environmental, animal and human sources . E. faecalis: . most common in the gastrointestinal tract, and may be found in human and animal faeces . associated with clinical urinary tract infections, hepatobiliary sepsis, endocarditis, surgical wound infection, bacteraemia and neonatal sepsis . able to survive a range of adverse environments allowing multiple routes of cross- contamination . resistant to a broad range of antibiotics including ampicillin, ciprofloxacin and imipenem ANTIBIOTICS NATURALLY OCCURING -

Gao-15-80, Anthrax
United States Government Accountability Office Report to Congressional Requesters December 2014 ANTHRAX Agency Approaches to Validation and Statistical Analyses Could Be Improved GAO-15-80 December 2014 ANTHRAX Agency Approaches to Validation and Statistical Analyses Could Be Improved Highlights of GAO-15-80, a report to congressional requesters Why GAO Did This Study What GAO Found In 2001, the FBI investigated an After the 2001 Anthrax attacks, the genetic tests that were conducted by the intentional release of B. anthracis, a Federal Bureau of Investigation’s (FBI) four contractors were generally bacterium that causes anthrax, which scientifically verified and validated, and met the FBI’s criteria. However, GAO was identified as the Ames strain. found that the FBI lacked a comprehensive approach—or framework—that could Subsequently, FBI contractors have ensured standardization of the testing process. As a result, each of the developed and validated several contractors developed their tests differently, and one contractor did not conduct genetic tests to analyze B. anthracis verification testing, a key step in determining whether a test will meet a user’s samples for the presence of certain requirements, such as for sensitivity or accuracy. Also, GAO found that the genetic mutations. The FBI had contractors did not develop the level of statistical confidence for interpreting the previously collected and maintained testing results for the validation tests they performed. Responses to future these samples in a repository. incidents could be improved by using a standardized framework for achieving GAO was asked to review the FBI’s minimum performance standards during verification and validation, and by genetic test development process and incorporating statistical analyses when interpreting validation testing results.