Gao-15-80, Anthrax
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Developing Drugs for Prophylaxis of Inhalational Anthrax Guidance for Industry
Anthrax: Developing Drugs for Prophylaxis of Inhalational Anthrax Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) May 2018 Clinical/Antimicrobial Anthrax: Developing Drugs for Prophylaxis of Inhalational Anthrax Guidance for Industry Additional copies are available from: Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration 10001 New Hampshire Ave., Hillandale Bldg., 4th Floor Silver Spring, MD 20993-0002 Phone: 855-543-3784 or 301-796-3400; Fax: 301-431-6353; Email: [email protected] https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) May 2018 Clinical/Antimicrobial TABLE OF CONTENTS I. INTRODUCTION............................................................................................................. 1 II. BACKGROUND ............................................................................................................... 2 A. Historical Background................................................................................................................... 2 B. Indication for Prophylaxis of Inhalational Anthrax ................................................................... 2 III. DEVELOPMENT PROGRAM ....................................................................................... 3 A. General -

Amerithrax Investigative Summary
The United States Department of Justice AMERITHRAX INVESTIGATIVE SUMMARY Released Pursuant to the Freedom of Information Act Friday, February 19, 2010 TABLE OF CONTENTS I. THE ANTHRAX LETTER ATTACKS . .1 II. EXECUTIVE SUMMARY . 4 A. Overview of the Amerithrax Investigation . .4 B. The Elimination of Dr. Steven J. Hatfill as a Suspect . .6 C. Summary of the Investigation of Dr. Bruce E. Ivins . 6 D. Summary of Evidence from the Investigation Implicating Dr. Ivins . .8 III. THE AMERITHRAX INVESTIGATION . 11 A. Introduction . .11 B. The Investigation Prior to the Scientific Conclusions in 2007 . 12 1. Early investigation of the letters and envelopes . .12 2. Preliminary scientific testing of the Bacillus anthracis spore powder . .13 3. Early scientific findings and conclusions . .14 4. Continuing investigative efforts . 16 5. Assessing individual suspects . .17 6. Dr. Steven J. Hatfill . .19 7. Simultaneous investigative initiatives . .21 C. The Genetic Analysis . .23 IV. THE EVIDENCE AGAINST DR. BRUCE E. IVINS . 25 A. Introduction . .25 B. Background of Dr. Ivins . .25 C. Opportunity, Access and Ability . 26 1. The creation of RMR-1029 – Dr. Ivins’s flask . .26 2. RMR-1029 is the source of the murder weapon . 28 3. Dr. Ivins’s suspicious lab hours just before each mailing . .29 4. Others with access to RMR-1029 have been ruled out . .33 5. Dr. Ivins’s considerable skill and familiarity with the necessary equipment . 36 D. Motive . .38 1. Dr. Ivins’s life’s work appeared destined for failure, absent an unexpected event . .39 2. Dr. Ivins was being subjected to increasing public criticism for his work . -

Investigation of Anthrax Associated with Intentional Exposure
October 19, 2001 / Vol. 50 / No. 41 889 Update: Investigation of Anthrax Associated with Intentional Exposure and Interim Public Health Guidelines, October 2001 893 Recognition of Illness Associated with the Intentional Release of a Biologic Agent 897 Weekly Update: West Nile Virus Activity — United States, October 10–16, 2001 Update: Investigation of Anthrax Associated with Intentional Exposure and Interim Public Health Guidelines, October 2001 On October 4, 2001, CDC and state and local public health authorities reported a case of inhalational anthrax in Florida (1 ). Additional cases of anthrax subsequently have been reported from Florida and New York City. This report updates the findings of these case investigations, which indicate that infections were caused by the intentional release of Bacillus anthracis. This report also includes interim guidelines for postexposure pro- phylaxis for prevention of inhalational anthrax and other information to assist epidemi- ologists, clinicians, and laboratorians responding to intentional anthrax exposures. For these investigations, a confirmed case of anthrax was defined as 1) a clinically compatible case of cutaneous, inhalational, or gastrointestinal illness* that is laboratory confirmed by isolation of B. anthracis from an affected tissue or site or 2) other labora- tory evidence of B. anthracis infection based on at least two supportive laboratory tests. A suspected case was defined as 1) a clinically compatible case of illness without isola- tion of B. anthracis and no alternative diagnosis, but with laboratory evidence of B. anthracis by one supportive laboratory test or 2) a clinically compatible case of an- thrax epidemiologically linked to a confirmed environmental exposure, but without cor- roborative laboratory evidence of B. -

2001 Anthrax Letters
Chapter Five: 2001 Anthrax Letters Author’s Note: The analysis and comments regarding the communication efforts described in this case study are solely those of the authors; this analysis does not represent the official position of the FDA. This case was selected because it is one of the few major federal efforts to distribute medical countermeasures in response to an acute biological incident. These events occurred more than a decade ago and represent the early stages of US biosecurity preparedness and response; however, this incident serves as an excellent illustration of the types of communication challenges expected in these scenarios. Due in part to the extended time since these events and the limited accessibility of individual communications and messages, this case study does not provide a comprehensive assessment of all communication efforts. In contrast to the previous case studies in this casebook, the FDA’s role in the 2001 anthrax response was relatively small, and as such, this analysis focuses principally on the communication efforts of the CDC and state and local public health agencies. The 2001 anthrax attacks have been studied extensively, and the myriad of internal and external assessments led to numerous changes to response and communications policies and protocols. The authors intend to use this case study as a means of highlighting communication challenges strictly within the context of this incident, not to evaluate the success or merit of changes made as a result of these events. Abstract The dissemination of Bacillus anthracis via the US Postal Service (USPS) in 2001 represented a new public health threat, the first intentional exposure to anthrax in the United States. -

Molecular Subtyping of Bacillus Anthracis and the 2001 Bioterrorism-Associated Anthrax Outbreak, United States Alex R
BIOTERRORISM-RELATED ANTHRAX Molecular Subtyping of Bacillus anthracis and the 2001 Bioterrorism-Associated Anthrax Outbreak, United States Alex R. Hoffmaster,* Collette C. Fitzgerald,* Efrain Ribot,* Leonard W. Mayer,* and Tanja Popovic* Molecular subtyping of Bacillus anthracis played an important role in differentiating and identifying strains during the 2001 bioterrorism-associated outbreak. Because B. anthracis has a low level of genetic variabil- ity, only a few subtyping methods, with varying reliability, exist. We initially used multiple-locus variable- number tandem repeat analysis (MLVA) to subtype 135 B. anthracis isolates associated with the outbreak. All isolates were determined to be of genotype 62, the same as the Ames strain used in laboratories. We sequenced the protective antigen gene (pagA) from 42 representative outbreak isolates and determined they all had a pagA sequence indistinguishable from the Ames strain (PA genotype I). MLVA and pagA sequencing were also used on DNA from clinical specimens, making subtyping B. anthracis possible with- out an isolate. Use of high-resolution molecular subtyping determined that all outbreak isolates were indis- tinguishable by the methods used and probably originated from a single source. In addition, subtyping rapidly identified laboratory contaminants and nonoutbreak–related isolates. he recent bioterrorism-associated anthrax outbreak dem- subtype 26 diverse B. anthracis isolates into six PA genotypes T onstrated the need for rapid molecular subtyping of Bacil- (8). Although sequencing of pagA results in limited numbers lus anthracis isolates. Numerous methods, including multiple- of subtypes, it does have the added benefit of determining if locus enzyme electrophoresis (MEE) and multiple-locus the pagA gene has been altered or engineered. -

Lessons from the Anthrax Attacks Implications for US
Lessons from the Anthrax Attacks Implications for US. Bioterrorism Preparedness A Report on a National Forum on Biodefense Author David Heymart Research Assistants Srusha Ac h terb erg, L Joelle Laszld Organized by the Center for Strategic and International Studies and the Defense Threat Reduction Agency --CFr”V? --.....a DlSTRf BUTION This report ISfor official use only; distribution authorized to U S. government agencies, designated contractors, and those with an official need Contains information that may be exempt from public release under the Freedom of Information Act. exemption number 2 (5 USC 552); exemption number 3 (’lo USC 130).Approvalof the Defense Threat Reduction Agency prior to public release is requrred Contract Number OTRAM-02-C-0013 For Official Use Only About CSIS For four decades, the Center for Stravgic and Internahonal Studies (CSIS)has been dedicated 10 providing world leaders with strategic insights on-and pohcy solubons tcurrentand emergtng global lssues CSIS IS led by John J Hamre, former L S deputy secretary of defense It is guided by a board of trustees chaired by former U S senator Sam Nunn and consistlng of prominent individuals horn both the public and private sectors The CSIS staff of 190 researchers and support staff focus pnrnardy on three subjecr areas First, CSIS addresses the fuU spectrum of new challenges to national and mternabonal security Second, it maintains resident experts on all of the world's myor geographical regions Third, it IS committed to helping to develop new methods of governance for the global age, to this end, CSIS has programs on technology and pubhc policy, International trade and finance, and energy Headquartered in Washington, D-C ,CSIS IS pnvate, bipartlsan, and tax-exempt CSIS docs not take specific policy positions, accordygly, all views expressed herein should be understood to be solely those ofthe author Spousor. -
Anthrax Reporting and Investigation Guideline
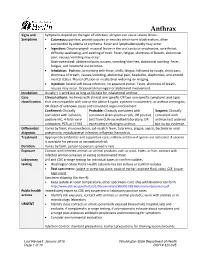
Anthrax Signs and Symptoms depend on the type of infection; all types can cause severe illness: Symptoms • Cutaneous: painless, pruritic papules or vesicles which form black eschars, often surrounded by edema or erythema. Fever and lymphadenopathy may occur. • Ingestion: Oropharyngeal: mucosal lesion in the oral cavity or oropharynx, sore throat, difficulty swallowing, and swelling of neck. Fever, fatigue, shortness of breath, abdominal pain, nausea/vomiting may occur. Gastrointestinal: abdominal pain, nausea, vomiting/diarrhea, abdominal swelling. Fever, fatigue, and headache are common. • Inhalation: Biphasic, presenting with fever, chills, fatigue, followed by cough, chest pain, shortness of breath, nausea/vomiting, abdominal pain, headache, diaphoresis, and altered mental status. Pleural effusion or mediastinal widening on imaging. • Injection: Severe soft tissue infection; no apparent eschar. Fever, shortness of breath, nausea may occur. Occasional meningeal or abdominal involvement. Incubation Usually < 1 week but as long as 60 days for inhalational anthrax Case Clinical criteria: An illness with at least one specific OR two non-specific symptoms and signs classification that are compatible with one of the above 4 types, systemic involvement, or anthrax meningitis; OR death of unknown cause and consistent organ involvement Confirmed: Clinically Probable: Clinically consistent with Suspect: Clinically consistent with isolation, consistent Gram-positive rods, OR positive consistent with positive IHC, 4-fold rise in test from CLIA-accredited laboratory, OR anthrax test ordered antibodies, PCR, or LF MS epi evidence relating to anthrax but no epi evidence Differential Varies by form; mononucleosis, cat-scratch fever, tularemia, plague, sepsis, bacterial or viral diagnosis pneumonia, mycobacterial infection, influenza, hantavirus Treatment Appropriate antibiotics and supportive care; anthrax antitoxin if spores are activated. -

Bioterrorism & Biodefense
Hugh-Jones et al. J Bioterr Biodef 2011, S3 Bioterrorism & Biodefense http://dx.doi.org/10.4172/2157-2526.S3-001 Review Article Open Access The 2001 Attack Anthrax: Key Observations Martin E Hugh-Jones1*, Barbara Hatch Rosenberg2 and Stuart Jacobsen3 1Professor Emeritus, Louisiana State University; Anthrax Moderator, ProMED-mail, USA 2Sloan-Kettering Institute for Cancer Research and State Univ. of NY-Purchase (retired); Scientists Working Group on CBW, Center for Arms Control and Non-Proliferation, USA 3Technical Consultant Silicon Materials, Dallas, TX,USA Abstract Unresolved scientificquestions, remaining ten years after the anthrax attacks, three years after the FBI accused a dead man of perpetrating the 2001 anthrax attacks singlehandedly, and more than a year since they closed the case without further investigation, indictment or trial, are perpetuating serious concerns that the FBI may have accused the wrong person of carrying out the anthrax attacks. The FBI has not produced concrete evidence on key questions: • Where and how were the anthrax spores in the attack letters prepared? There is no material evidence of where the attack anthrax was made, and no direct evidence that any specific individual made the anthrax, or mailed it. On the basis of a number` of assumptions, the FBI has not scrutinized the most likely laboratories. • How and why did the spore powders acquire the high levels of silicon and tin found in them? The FBI has repeatedly insisted that the powders in the letters contained no additives, but they also claim that they have not been able to reproduce the high silicon content in the powders, and there has been little public mention of the extraordinary presence of tin. -

Bioterrorism, Biological Weapons and Anthrax
Bioterrorism, Biological Weapons and Anthrax Part IV Written by Arthur H. Garrison Criminal Justice Planning Coordinator Delaware Criminal Justice Council Bioterrorism and biological weapons The use of bio-terrorism and bio-warfare dates back to 6th century when the Assyrians poisoned the well water of their enemies. The goal of using biological weapons is to cause massive sickness or death in the intended target. Bioterrorism and biological weapons The U.S. took the threat of biological weapons attack seriously after Gulf War. Anthrax vaccinations of U.S. troops Investigating Iraq and its biological weapons capacity The Soviet Union manufactured various types of biological weapons during the 1980’s • To be used after a nuclear exchange • Manufacturing new biological weapons – Gene engineering – creating new types of viruses/bacteria • Contagious viruses – Ebola, Marburg (Filoviruses) - Hemorrhagic fever diseases (vascular system dissolves) – Smallpox The spread of biological weapons after the fall of the Soviet Union •Material • Knowledge and expertise •Equipment Bioterrorism and biological weapons There are two basic categories of biological warfare agents. Microorganisms • living organic germs, such as anthrax (bacillus anthrax). –Bacteria –Viruses Toxins • By-products of living organisms (natural poisons) such as botulism (botulinum toxin) which is a by- product of growing the microorganism clostridium botulinum Bioterrorism and biological weapons The U.S. was a leader in the early research on biological weapons Research on making -

Antibacterial Photosensitization Through Activation of PNAS PLUS Coproporphyrinogen Oxidase
Antibacterial photosensitization through activation of PNAS PLUS coproporphyrinogen oxidase Matthew C. Surdela, Dennis J. Horvath Jr.a, Lisa J. Lojeka, Audra R. Fullena, Jocelyn Simpsona, Brendan F. Dutterb,c, Kenneth J. Sallenga, Jeremy B. Fordd, J. Logan Jenkinsd, Raju Nagarajane, Pedro L. Teixeiraf, Matthew Albertollec,g, Ivelin S. Georgieva,e,h, E. Duco Jansend, Gary A. Sulikowskib,c, D. Borden Lacya,d, Harry A. Daileyi,j,k, and Eric P. Skaara,1 aDepartment of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, Nashville, TN 37232; bDepartment of Chemistry, Vanderbilt University, Nashville, TN 37232; cVanderbilt Institute for Chemical Biology, Nashville, TN 37232; dDepartment of Biomedical Engineering, Vanderbilt University, Nashville, TN 37232; eVanderbilt Vaccine Center, Vanderbilt University Medical Center, Nashville, TN 37232; fBiomedical Informatics, Vanderbilt University School of Medicine, Nashville, TN 37203; gDepartment of Biochemistry, Vanderbilt University, Nashville, TN 37232; hDepartment of Electrical Engineering and Computer Science, Vanderbilt University, Nashville, TN 37232; iBiomedical and Health Sciences Institute, University of Georgia, Athens, GA 30602; jDepartment of Microbiology, University of Georgia, Athens, GA 30602; and kDepartment of Biochemistry and Molecular Biology, University of Georgia, Athens, GA 30602 Edited by Ferric C. Fang, University of Washington School of Medicine, Seattle, WA, and accepted by Editorial Board Member Carl F. Nathan June 26, 2017 (received for review January 10, 2017) Gram-positive bacteria cause the majority of skin and soft tissue Small-molecule VU0038882 (‘882) was previously identified in infections (SSTIs), resulting in the most common reason for clinic a screen for activators of the S. aureus heme-sensing system two- visits in the United States. -

Vaccination of Rhesus Macaques with the Anthrax Vaccine Adsorbed
CLINICAL AND VACCINE IMMUNOLOGY, Nov. 2010, p. 1753–1762 Vol. 17, No. 11 1556-6811/10/$12.00 doi:10.1128/CVI.00174-10 Copyright © 2010, American Society for Microbiology. All Rights Reserved. Vaccination of Rhesus Macaques with the Anthrax Vaccine Adsorbed Vaccine Produces a Serum Antibody Response That Effectively Neutralizes Receptor-Bound Protective Antigen In Vitroᰔ Kristin H. Clement,1* Thomas L. Rudge, Jr.,1 Heather J. Mayfield,1 Lena A. Carlton,1 Arelis Hester,1 Nancy A. Niemuth,1 Carol L. Sabourin,1 April M. Brys,1 and Conrad P. Quinn2 Battelle Memorial Institute, 505 King Avenue, Columbus, Ohio 43201,1 and Meningitis and Vaccine Preventable Diseases Branch, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia 303332 Received 29 April 2010/Returned for modification 17 June 2010/Accepted 19 August 2010 Anthrax toxin (ATx) is composed of the binary exotoxins lethal toxin (LTx) and edema toxin (ETx). They have separate effector proteins (edema factor and lethal factor) but have the same binding protein, protective antigen (PA). PA is the primary immunogen in the current licensed vaccine anthrax vaccine adsorbed (AVA [BioThrax]). AVA confers protective immunity by stimulating production of ATx-neutralizing antibodies, which could block the intoxication process at several steps (binding of PA to the target cell surface, furin cleavage, toxin complex formation, and binding/translocation of ATx into the cell). To evaluate ATx neutral- ization by anti-AVA antibodies, we developed two low-temperature LTx neutralization activity (TNA) assays that distinguish antibody blocking before and after binding of PA to target cells (noncomplexed [NC] and receptor-bound [RB] TNA assays). -

Anti-Anthrax Agents Means That New Antibiotics Are Needed to the 14-Membered Ring
research highlights NATURAL PRODUCTS infection and its resistance to treatment system — along with a dichloro group on Anti-anthrax agents means that new antibiotics are needed to the 14-membered ring. Fenical and the Angew. Chem. Int. Ed. 52, 7822–7824 (2013) target this bacterium. team decided to incorporate this dichloro In an effort to discover new antimicrobial moiety into anthracimycin to see what H H scaffolds, a team led by William Fenical at the effect this would have on the antimicrobial University of California at San Diego, USA, activity. Interestingly, the dichloro analogue H H H isolated metabolites from a marine organism was approximately half as potent against H H Cl H (a species of Streptomyces) and tested them Bacillus anthracis, however, it exhibited O O O O Cl for antibacterial activity. One metabolite greater activity against some strains of O O H O O showed potent activity against the Gram- Gram-negative bacteria — which the team positive Bacillus anthracis and methicillin- believe is linked to easier penetration Anthracimycin Dichloro analogue resistant Staphylococcus aureus; although through the cell wall. RJ it had only very limited activity against Few diseases carry the sinister connotations Gram-negative bacteria such as Escherichia REACTIVE INTERMEDIATES of anthrax. It is a deadly infection that coli. A series of NMR spectroscopy, mass Non-classical crystals predominantly affects herbivores, although spectrometry and X-ray crystallography Science 341, 62–64 (2013) it has also been used for bioterrorism analyses enabled the team to characterize and adapted for biowarfare. Anthrax is the structure and stereochemistry of this When either enantiomer of exo-2-norbornyl caused by Bacillus anthracis, and, like other new antimicrobial compound — which they bromide reacts with a nucleophile, a racemic bacterial diseases, it is normally treated with termed anthracimycin.