Investigation of Anthrax Associated with Intentional Exposure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toxic Shock Syndrome Contributors: Noah Craft MD, Phd, Lindy P
** no patient handout Toxic shock syndrome Contributors: Noah Craft MD, PhD, Lindy P. Fox MD, Lowell A. Goldsmith MD, MPH SynopsisToxic shock syndrome (TSS) is a severe exotoxin-mediated bacterial infection that is characterized by high fevers, headache, pharyngitis, vomiting, diarrhea, and hypotension. Two subtypes of TSS exist, based on the bacterial etiology: Staphylococcus aureus and group A streptococci. Significantly, the severity of TSS can range from mild disease to rapid progression to shock and end organ failure. The dermatologic manifestations of TSS include the following: • Erythema of the palms and soles that desquamates 1-3 weeks after the initial onset • Diffuse scarlatiniform exanthem that begins on the trunk and spreads toward the extremities • Erythema of the mucous membranes (strawberry tongue and conjunctival hyperemia) TSS was identified in and most commonly affected menstruating young white females using tampons in the 1980s. Current TSS cases are seen in post-surgical interventions in men, women, and children, as well as in other settings, in addition to cases of menstrual TSS, which have declined with increased public education on tampon usage and TSS. One study in Japanese patients found the highest TSS incidence to occur among children with burns, as Staphylococcus colonization is high in this subgroup and antibody titers are not yet sufficient to protect children from the exotoxins causing TSS. Staphylococcal TSS is caused by S. aureus strains that can produce the toxic shock syndrome toxin-1 (TSST-1). TSST-1 is believed to cause disease via direct effects on end organs, impairing clearance of gut flora derived endotoxins, with TSST-1 acting as a superantigen leading to massive nonspecific activation of T-cells and subsequent inflammation and vascular leakage. -

Amerithrax Investigative Summary
The United States Department of Justice AMERITHRAX INVESTIGATIVE SUMMARY Released Pursuant to the Freedom of Information Act Friday, February 19, 2010 TABLE OF CONTENTS I. THE ANTHRAX LETTER ATTACKS . .1 II. EXECUTIVE SUMMARY . 4 A. Overview of the Amerithrax Investigation . .4 B. The Elimination of Dr. Steven J. Hatfill as a Suspect . .6 C. Summary of the Investigation of Dr. Bruce E. Ivins . 6 D. Summary of Evidence from the Investigation Implicating Dr. Ivins . .8 III. THE AMERITHRAX INVESTIGATION . 11 A. Introduction . .11 B. The Investigation Prior to the Scientific Conclusions in 2007 . 12 1. Early investigation of the letters and envelopes . .12 2. Preliminary scientific testing of the Bacillus anthracis spore powder . .13 3. Early scientific findings and conclusions . .14 4. Continuing investigative efforts . 16 5. Assessing individual suspects . .17 6. Dr. Steven J. Hatfill . .19 7. Simultaneous investigative initiatives . .21 C. The Genetic Analysis . .23 IV. THE EVIDENCE AGAINST DR. BRUCE E. IVINS . 25 A. Introduction . .25 B. Background of Dr. Ivins . .25 C. Opportunity, Access and Ability . 26 1. The creation of RMR-1029 – Dr. Ivins’s flask . .26 2. RMR-1029 is the source of the murder weapon . 28 3. Dr. Ivins’s suspicious lab hours just before each mailing . .29 4. Others with access to RMR-1029 have been ruled out . .33 5. Dr. Ivins’s considerable skill and familiarity with the necessary equipment . 36 D. Motive . .38 1. Dr. Ivins’s life’s work appeared destined for failure, absent an unexpected event . .39 2. Dr. Ivins was being subjected to increasing public criticism for his work . -

Toxic Shock-Like Syndrome Associated with Necrotizing Streptococcus Pyogenes Infection
Henry Ford Hospital Medical Journal Volume 37 Number 2 Article 5 6-1989 Toxic Shock-like Syndrome Associated with Necrotizing Streptococcus Pyogenes Infection Thomas J. Connolly Donald J. Pavelka Eugene F. Lanspa Thomas L. Connolly Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Connolly, Thomas J.; Pavelka, Donald J.; Lanspa, Eugene F.; and Connolly, Thomas L. (1989) "Toxic Shock- like Syndrome Associated with Necrotizing Streptococcus Pyogenes Infection," Henry Ford Hospital Medical Journal : Vol. 37 : No. 2 , 69-72. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol37/iss2/5 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. Toxic Shock-like Syndrome Associated with Necrotizing Streptococcus Pyogenes Infection Thomas J. Connolly,* Donald J. Pavelka, MD,^ Eugene F. Lanspa, MD, and Thomas L. Connolly, MD' Two patients with toxic shock-like syndrome are presented. Bolh patients had necrotizing cellulitis due to Streptococcus pyogenes, and both patients required extensive surgical debridement. The association of Streptococcus pyogenes infection and toxic shock-like syndrome is discussed. (Henry Ford Hosp MedJ 1989:37:69-72) ince 1978, toxin-producing strains of Staphylococcus brought to the emergency room where a physical examination revealed S aureus have been implicated as the cause of the toxic shock a temperature of 40.9°C (I05.6°F), blood pressure of 98/72 mm Hg, syndrome (TSS), which is characterized by fever and rash and respiration of 36 breaths/min, and a pulse of 72 beats/min. -

2001 Anthrax Letters
Chapter Five: 2001 Anthrax Letters Author’s Note: The analysis and comments regarding the communication efforts described in this case study are solely those of the authors; this analysis does not represent the official position of the FDA. This case was selected because it is one of the few major federal efforts to distribute medical countermeasures in response to an acute biological incident. These events occurred more than a decade ago and represent the early stages of US biosecurity preparedness and response; however, this incident serves as an excellent illustration of the types of communication challenges expected in these scenarios. Due in part to the extended time since these events and the limited accessibility of individual communications and messages, this case study does not provide a comprehensive assessment of all communication efforts. In contrast to the previous case studies in this casebook, the FDA’s role in the 2001 anthrax response was relatively small, and as such, this analysis focuses principally on the communication efforts of the CDC and state and local public health agencies. The 2001 anthrax attacks have been studied extensively, and the myriad of internal and external assessments led to numerous changes to response and communications policies and protocols. The authors intend to use this case study as a means of highlighting communication challenges strictly within the context of this incident, not to evaluate the success or merit of changes made as a result of these events. Abstract The dissemination of Bacillus anthracis via the US Postal Service (USPS) in 2001 represented a new public health threat, the first intentional exposure to anthrax in the United States. -

Detection of ESKAPE Pathogens and Clostridioides Difficile in Simulated
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.04.433847; this version posted March 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Detection of ESKAPE pathogens and Clostridioides difficile in 2 Simulated Skin Transmission Events with Metagenomic and 3 Metatranscriptomic Sequencing 4 5 Krista L. Ternusa#, Nicolette C. Keplingera, Anthony D. Kappella, Gene D. Godboldb, Veena 6 Palsikara, Carlos A. Acevedoa, Katharina L. Webera, Danielle S. LeSassiera, Kathleen Q. 7 Schultea, Nicole M. Westfalla, and F. Curtis Hewitta 8 9 aSignature Science, LLC, 8329 North Mopac Expressway, Austin, Texas, USA 10 bSignature Science, LLC, 1670 Discovery Drive, Charlottesville, VA, USA 11 12 #Address correspondence to Krista L. Ternus, [email protected] 13 14 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.04.433847; this version posted March 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 15 1 Abstract 16 Background: Antimicrobial resistance is a significant global threat, posing major public health 17 risks and economic costs to healthcare systems. Bacterial cultures are typically used to diagnose 18 healthcare-acquired infections (HAI); however, culture-dependent methods provide limited 19 presence/absence information and are not applicable to all pathogens. -

Lessons from the Anthrax Attacks Implications for US
Lessons from the Anthrax Attacks Implications for US. Bioterrorism Preparedness A Report on a National Forum on Biodefense Author David Heymart Research Assistants Srusha Ac h terb erg, L Joelle Laszld Organized by the Center for Strategic and International Studies and the Defense Threat Reduction Agency --CFr”V? --.....a DlSTRf BUTION This report ISfor official use only; distribution authorized to U S. government agencies, designated contractors, and those with an official need Contains information that may be exempt from public release under the Freedom of Information Act. exemption number 2 (5 USC 552); exemption number 3 (’lo USC 130).Approvalof the Defense Threat Reduction Agency prior to public release is requrred Contract Number OTRAM-02-C-0013 For Official Use Only About CSIS For four decades, the Center for Stravgic and Internahonal Studies (CSIS)has been dedicated 10 providing world leaders with strategic insights on-and pohcy solubons tcurrentand emergtng global lssues CSIS IS led by John J Hamre, former L S deputy secretary of defense It is guided by a board of trustees chaired by former U S senator Sam Nunn and consistlng of prominent individuals horn both the public and private sectors The CSIS staff of 190 researchers and support staff focus pnrnardy on three subjecr areas First, CSIS addresses the fuU spectrum of new challenges to national and mternabonal security Second, it maintains resident experts on all of the world's myor geographical regions Third, it IS committed to helping to develop new methods of governance for the global age, to this end, CSIS has programs on technology and pubhc policy, International trade and finance, and energy Headquartered in Washington, D-C ,CSIS IS pnvate, bipartlsan, and tax-exempt CSIS docs not take specific policy positions, accordygly, all views expressed herein should be understood to be solely those ofthe author Spousor. -

Infective Endocarditis Caused by C. Sordellii: the First Case Report from India
Published online: 2021-05-19 THIEME 74 C.Case sordellii Report in Endocarditis Chaudhry et al. Infective Endocarditis Caused by C. sordellii: The First Case Report from India Rama Chaudhry1 Tej Bahadur1 Tanu Sagar1 Sonu Kumari Agrawal1 Nazneen Arif1 Shiv K. Choudhary2 Nishant Verma1 1Department of Microbiology, All India Institute of Medical Address for correspondence Dr. Rama Chaudhry, MD, Department Sciences, New Delhi, India of Microbiology, All India Institute of Medical Sciences, Ansari Nagar, 2Department of Cardiothoracic and Vascular Surgery, All India New Delhi 110029, India (e-mail: [email protected]). Institute of Medical Sciences, New Delhi, India J Lab Physicians 2021;13:74–76. Abstract Clostridium sordellii is a gram-positive anaerobic bacteria most commonly isolated Keywords from skin and soft tissue infection, penetrating injurious and intravenous drug abus- ► infective endocarditis ers. The exotoxins produced by the bacteria are associated with toxic shock syndrome. ► Clostridium sordellii We report here a first case of infective endocarditis due to C. sordellii from a female ► diagnosis patient with ventricular septal defect from India. Introduction admitted to the cardiothoracic vascular surgery ward of All India Institute of Medical Sciences (AIIMS), New Delhi, India Anaerobes are major components of the normal micro- with complaints of worsening shortness of breath and pal- bial flora present on human skin and mucosa. Infections pitations. Patient reported having an episode of IE 3 months due to anaerobic bacteria are common, but they are dif- back for which she had been admitted to AIIMS and was ficult to isolate from infected sites and are often over- discharged after treatment. -
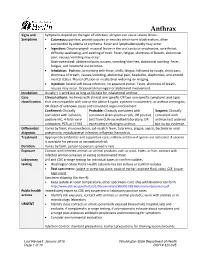
Anthrax Reporting and Investigation Guideline
Anthrax Signs and Symptoms depend on the type of infection; all types can cause severe illness: Symptoms • Cutaneous: painless, pruritic papules or vesicles which form black eschars, often surrounded by edema or erythema. Fever and lymphadenopathy may occur. • Ingestion: Oropharyngeal: mucosal lesion in the oral cavity or oropharynx, sore throat, difficulty swallowing, and swelling of neck. Fever, fatigue, shortness of breath, abdominal pain, nausea/vomiting may occur. Gastrointestinal: abdominal pain, nausea, vomiting/diarrhea, abdominal swelling. Fever, fatigue, and headache are common. • Inhalation: Biphasic, presenting with fever, chills, fatigue, followed by cough, chest pain, shortness of breath, nausea/vomiting, abdominal pain, headache, diaphoresis, and altered mental status. Pleural effusion or mediastinal widening on imaging. • Injection: Severe soft tissue infection; no apparent eschar. Fever, shortness of breath, nausea may occur. Occasional meningeal or abdominal involvement. Incubation Usually < 1 week but as long as 60 days for inhalational anthrax Case Clinical criteria: An illness with at least one specific OR two non-specific symptoms and signs classification that are compatible with one of the above 4 types, systemic involvement, or anthrax meningitis; OR death of unknown cause and consistent organ involvement Confirmed: Clinically Probable: Clinically consistent with Suspect: Clinically consistent with isolation, consistent Gram-positive rods, OR positive consistent with positive IHC, 4-fold rise in test from CLIA-accredited laboratory, OR anthrax test ordered antibodies, PCR, or LF MS epi evidence relating to anthrax but no epi evidence Differential Varies by form; mononucleosis, cat-scratch fever, tularemia, plague, sepsis, bacterial or viral diagnosis pneumonia, mycobacterial infection, influenza, hantavirus Treatment Appropriate antibiotics and supportive care; anthrax antitoxin if spores are activated. -

Bioterrorism, Biological Weapons and Anthrax
Bioterrorism, Biological Weapons and Anthrax Part IV Written by Arthur H. Garrison Criminal Justice Planning Coordinator Delaware Criminal Justice Council Bioterrorism and biological weapons The use of bio-terrorism and bio-warfare dates back to 6th century when the Assyrians poisoned the well water of their enemies. The goal of using biological weapons is to cause massive sickness or death in the intended target. Bioterrorism and biological weapons The U.S. took the threat of biological weapons attack seriously after Gulf War. Anthrax vaccinations of U.S. troops Investigating Iraq and its biological weapons capacity The Soviet Union manufactured various types of biological weapons during the 1980’s • To be used after a nuclear exchange • Manufacturing new biological weapons – Gene engineering – creating new types of viruses/bacteria • Contagious viruses – Ebola, Marburg (Filoviruses) - Hemorrhagic fever diseases (vascular system dissolves) – Smallpox The spread of biological weapons after the fall of the Soviet Union •Material • Knowledge and expertise •Equipment Bioterrorism and biological weapons There are two basic categories of biological warfare agents. Microorganisms • living organic germs, such as anthrax (bacillus anthrax). –Bacteria –Viruses Toxins • By-products of living organisms (natural poisons) such as botulism (botulinum toxin) which is a by- product of growing the microorganism clostridium botulinum Bioterrorism and biological weapons The U.S. was a leader in the early research on biological weapons Research on making -

Antibacterial Photosensitization Through Activation of PNAS PLUS Coproporphyrinogen Oxidase
Antibacterial photosensitization through activation of PNAS PLUS coproporphyrinogen oxidase Matthew C. Surdela, Dennis J. Horvath Jr.a, Lisa J. Lojeka, Audra R. Fullena, Jocelyn Simpsona, Brendan F. Dutterb,c, Kenneth J. Sallenga, Jeremy B. Fordd, J. Logan Jenkinsd, Raju Nagarajane, Pedro L. Teixeiraf, Matthew Albertollec,g, Ivelin S. Georgieva,e,h, E. Duco Jansend, Gary A. Sulikowskib,c, D. Borden Lacya,d, Harry A. Daileyi,j,k, and Eric P. Skaara,1 aDepartment of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, Nashville, TN 37232; bDepartment of Chemistry, Vanderbilt University, Nashville, TN 37232; cVanderbilt Institute for Chemical Biology, Nashville, TN 37232; dDepartment of Biomedical Engineering, Vanderbilt University, Nashville, TN 37232; eVanderbilt Vaccine Center, Vanderbilt University Medical Center, Nashville, TN 37232; fBiomedical Informatics, Vanderbilt University School of Medicine, Nashville, TN 37203; gDepartment of Biochemistry, Vanderbilt University, Nashville, TN 37232; hDepartment of Electrical Engineering and Computer Science, Vanderbilt University, Nashville, TN 37232; iBiomedical and Health Sciences Institute, University of Georgia, Athens, GA 30602; jDepartment of Microbiology, University of Georgia, Athens, GA 30602; and kDepartment of Biochemistry and Molecular Biology, University of Georgia, Athens, GA 30602 Edited by Ferric C. Fang, University of Washington School of Medicine, Seattle, WA, and accepted by Editorial Board Member Carl F. Nathan June 26, 2017 (received for review January 10, 2017) Gram-positive bacteria cause the majority of skin and soft tissue Small-molecule VU0038882 (‘882) was previously identified in infections (SSTIs), resulting in the most common reason for clinic a screen for activators of the S. aureus heme-sensing system two- visits in the United States. -

Botulinum Toxin Ricin Toxin Staph Enterotoxin B
Botulinum Toxin Ricin Toxin Staph Enterotoxin B Source Source Source Clostridium botulinum, a large gram- Ricinus communis . seeds commonly called .Staphylococcus aureus, a gram-positive cocci positive, spore-forming, anaerobic castor beans bacillus Characteristics Characteristics .Appears as grape-like clusters on Characteristics .Toxin can be disseminated in the form of a Gram stain or as small off-white colonies .Grows anaerobically on Blood Agar and liquid, powder or mist on Blood Agar egg yolk plates .Toxin-producing and non-toxigenic strains Pathogenesis of S. aureus will appear morphologically Pathogenesis .A-chain inactivates ribosomes, identical interrupting protein synthesis .Toxin enters nerve terminals and blocks Pathogenesis release of acetylcholine, blocking .B-chain binds to carbohydrate receptors .Staphylococcus Enterotoxin B (SEB) is a neuro-transmission and resulting in on the cell surface and allows toxin superantigen. Toxin binds to human class muscle paralysis complex to enter cell II MHC molecules causing cytokine Toxicity release and system-wide inflammation Toxicity .Highly toxic by inhalation, ingestion Toxicity .Most lethal of all toxic natural substances and injection .Toxic by inhalation or ingestion .Groups A, B, E (rarely F) cause illness in .Less toxic by ingestion due to digestive humans activity and poor absorption Symptoms .Low dermal toxicity .4-10 h post-ingestion, 3-12 h post-inhalation Symptoms .Flu-like symptoms, fever, chills, .24-36 h (up to 3 d for wound botulism) Symptoms headache, myalgia .Progressive skeletal muscle weakness .18-24 h post exposure .Nausea, vomiting, and diarrhea .Symmetrical descending flaccid paralysis .Fever, cough, chest tightness, dyspnea, .Nonproductive cough, chest pain, .Can be confused with stroke, Guillain- cyanosis, gastroenteritis and necrosis; and dyspnea Barre syndrome or myasthenia gravis death in ~72 h .SEB can cause toxic shock syndrome + + + Gram stain Lipase on Ricin plant Castor beans S. -

Toxic Shock Syndrome
Iowa Department of Public Health TOXIC SHOCK SYNDROME Also known as: TSS Responsibilities: Hospital: Report by IDSS, facsimile, mail, or phone. Infection Preventionist: Report by IDSS, facsimile, mail, or phone. Assists with case investigation Lab: Report by IDSS, facsimile, mail, or phone. Physician: Report by facsimile, mail, or phone. Local Public Health Agency(LPHA): Report by IDSS, facsimile, mail, or phone. Iowa Department of Public Health Disease Reporting Hotline: (800) 362-2736 Secure Fax: (515) 281-5698 1) THE DISEASE AND ITS EPIDEMIOLOGY A. Agent Toxic shock syndrome (TSS) is a serious complication of infection with strains of Staphylococcus aureus that produce TSS toxin-1 (TSST-1) or strains of Streptococcus pyogenes that produce pyrogenic exotoxin A. S. pyogenes is more commonly known as group A streptococcus (GAS). B. Clinical Description TSS is a severe toxin-mediated illness with sudden onset of high fever, vomiting, profuse watery diarrhea, and myalgia, followed by hypotension and potentially shock. During the acute phase of the illness, a “sunburn-like” rash is present. One to two weeks after onset, desquamation of the skin occurs, especially on the soles and palms. Typically, the fever is higher than 102°F, the systolic blood pressure is <90 mm Hg and three or more of the following organ systems are involved: • gastrointestinal, • muscular, • mucous membranes (including vagina, pharynx, conjuctiva), • renal, • hepatic, • respiratory, • hematologic, or • central nervous system. Blood, cerebrospinal fluid and throat cultures are negative for pathogens other than S. aureus or GAS. Rocky Mountain spotted fever, leptospirosis and measles should be ruled out. TSS can be fatal.