Control and Management of Avian Tuberculosis in a Captive Collection of Wildfowl at the Wildfowl and Wetlands Trust Centre in Llanelli, Using an Enzyme-Linked Immunosorbent
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -

Parallel Evolution in the Major Haemoglobin Genes of Eight Species of Andean Waterfowl
Molecular Ecology (2009) doi: 10.1111/j.1365-294X.2009.04352.x Parallel evolution in the major haemoglobin genes of eight species of Andean waterfowl K. G. M C CRACKEN,* C. P. BARGER,* M. BULGARELLA,* K. P. JOHNSON,† S. A. SONSTHAGEN,* J. TRUCCO,‡ T. H. VALQUI,§– R. E. WILSON,* K. WINKER* and M. D. SORENSON** *Institute of Arctic Biology, Department of Biology and Wildlife, and University of Alaska Museum, University of Alaska Fairbanks, Fairbanks, AK 99775, USA, †Illinois Natural History Survey, Champaign, IL 61820, USA, ‡Patagonia Outfitters, Perez 662, San Martin de los Andes, Neuque´n 8370, Argentina, §Centro de Ornitologı´a y Biodiversidad (CORBIDI), Sta. Rita 117, Urbana Huertos de San Antonio, Surco, Lima 33, Peru´, –Museum of Natural Science, Louisiana State University, Baton Rouge, LA 70803, USA, **Department of Biology, Boston University, Boston, MA 02215, USA Abstract Theory predicts that parallel evolution should be common when the number of beneficial mutations is limited by selective constraints on protein structure. However, confirmation is scarce in natural populations. Here we studied the major haemoglobin genes of eight Andean duck lineages and compared them to 115 other waterfowl species, including the bar-headed goose (Anser indicus) and Abyssinian blue-winged goose (Cyanochen cyanopterus), two additional species living at high altitude. One to five amino acid replacements were significantly overrepresented or derived in each highland population, and parallel substitutions were more common than in simulated sequences evolved under a neutral model. Two substitutions evolved in parallel in the aA subunit of two (Ala-a8) and five (Thr-a77) taxa, and five identical bA subunit substitutions were observed in two (Ser-b4, Glu-b94, Met-b133) or three (Ser-b13, Ser-b116) taxa. -

Notes on Some Argentine Anatids
NOTESON SOME ARGENTINEANATIDS' MILTON W. WELLER ROM mid-August, 1964, until late July, 1965, I was engaged in field work F in Argentina studying waterfowl. Although special emphasis was placed on the Black-headed Duck (Heteronetta atricapilla) , 28 species of anatids were observed in various parts of Argentina. Because so little is known of these species, some general observations are summarized and discussed in the hope that it will point out gaps in our knowledge and encourage additional work on this interesting group. Field work was financed by Grant GB-1067 from the National Science Foundation. Studies of museum specimens in the United States were financed by a Chapman Grant of the American Museum of Natural History, and aided materially in appraising the significance of plumage sequences in Neotropical ducks. AQUATIC HABITATS Although the distribution of Argentine birds was considered by Dabbene (1910) and by Olrog (19591, little comment has been made on the distribution of water types and their influence on waterfowl distribution. Some helpful botanical comments are given by Cabrera (1953) for the Buenos Aires region and, Wetmores’ (1926) observations on both botany and ornithology are excellent. Although time did not permit detailed botanical work during this study, some obvious differences in life-form of marsh vegetation were recorded in the areas visited (Fig. 1) in relation to the species composi- tion of waterbirds. The most extensive zone of freshwater and semipermanent marshes is found in an area roughly bordered by the cities of Venado Tuerto (Santa Fe), Buenos Aires, General Lavalle, Mar de1 Plats and Azul (Buenos Aires). -

Bolivia: Endemic Macaws & More!
BOLIVIA: ENDEMIC MACAWS & MORE! PART II: FOOTHILLS, CLOUDFORESTS & THE ALTIPLANO SEPTEMBER 28–OCTOBER 8, 2018 Male Versicolored Barbet – Photo Andrew Whittaker LEADERS: ANDREW WHITTAKER & JULIAN VIDOZ LIST COMPILED BY: ANDREW WHITTAKER VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM Bolivia continued to exceed expectations on Part 2 of our tour! Steadily climbing up into the mighty ceiling of South America that is the Andes, we enjoyed exploring many more new, different, and exciting unspoiled bird-rich habitats, including magical Yungas cloudforest stretching as far as the eye could see; dry and humid Puna; towering snow-capped Andean peaks; vast stretches of Altiplano with its magical brackish lakes filled with immense numbers of glimmering flamingoes, and one of my favorite spots, the magnificent and famous Lake Titicaca (with its own flightless grebe). An overdose of stunning Andean scenery combined with marvelous shows of flowering plants enhanced our explorations of a never-ending array of different and exciting microhabitats for so many special and interesting Andean birds. We were rewarded with a fabulous trip record total of 341 bird species! Combining our two exciting Bolivia tours (Parts 1 and 2) gave us an all-time VENT record, an incredible grand total of 656 different bird species and 15 mammals! A wondrous mirage of glimmering pink hues of all three species of flamingos on the picturesque Bolivian Altiplano – Photo Andrew Whittaker Stunning Andes of Bolivia near Soroto on a clear day of our 2016 trip – Photo Andrew Whittaker Victor Emanuel Nature Tours 2 Bolivia Part 2, 2018 We began this second part of our Bolivian bird bonanza in the bustling city of Cochabamba, spending a fantastic afternoon birding the city’s rich lakeside in lovely late afternoon sun. -

Ducks, Geese, and Swans of the World by Paul A
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Ducks, Geese, and Swans of the World by Paul A. Johnsgard Papers in the Biological Sciences 2010 Ducks, Geese, and Swans of the World: Index Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/biosciducksgeeseswans Part of the Ornithology Commons Johnsgard, Paul A., "Ducks, Geese, and Swans of the World: Index" (2010). Ducks, Geese, and Swans of the World by Paul A. Johnsgard. 19. https://digitalcommons.unl.edu/biosciducksgeeseswans/19 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Ducks, Geese, and Swans of the World by Paul A. Johnsgard by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Index The following index is limited to the species of Anatidae; species of other bird families are not indexed, nor are subspecies included. However, vernacular names applied to certain subspecies that sometimes are considered full species are included, as are some generic names that are not utilized in this book but which are still sometimes applied to par ticular species or species groups. Complete indexing is limited to the entries that correspond to the vernacular names utilized in this book; in these cases the primary species account is indicated in italics. Other vernacular or scientific names are indexed to the section of the principal account only. Abyssinian blue-winged goose. See atratus, Cygnus, 31 Bernier teal. See Madagascan teal blue-winged goose atricapilla, Heteronetta, 365 bewickii, Cygnus, 44 acuta, Anas, 233 aucklandica, Anas, 214 Bewick swan, 38, 43, 44-47; PI. -

Machu Picchu & Abra Malaga, Peru II 2018 BIRDS
Field Guides Tour Report Machu Picchu & Abra Malaga, Peru II 2018 Oct 5, 2018 to Oct 14, 2018 Jesse Fagan For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. Our hike to the ridge above Abra Malaga couldn't have been more magical. Cerro Veronica in the background and feeding alpaca in the foreground, that is the Andes in Peru. Video grab by guide Jesse Fagan. I hope that you found Peru to be overwhelmingly beautiful. The food, the people, the Andes, the humid forest, Machu Picchu, and, of course, the birds. Indeed, this is my second home, and so I hope you felt welcomed here and decide to return soon. Peru is big (really big!) and there is much, much more to see. The birding was very good and a few highlights stood out for everyone. These included Andean Motmot, Pearled Treerunner, Plumbeous Rail, Versicolored Barbet, and Spectacled Redstart. However, a majority of the group thought seeing Andean Condors, well, in the Andes (!) was pretty darn cool. Black-faced Ibis feeding in plowed fields against Huaypo lake was a memory for a few others. I was glad to see one of my favorite birds stood out, the shocking Bearded Mountaineer (plus, maybe I am just partial to the "bearded" part). But you couldn't beat Andean Cock-of-the-Rock for shock value and the female on a nest was something you don't see everyday. Thanks to our team of drivers, and Lucrecia, our informative and always pleasant local guide. I hope to see you again soon. -
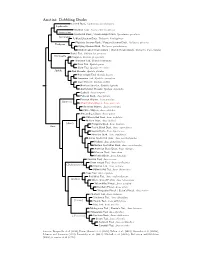
Anatini Tree
Anatini: Dabbling Ducks Crested Duck, Lophonetta specularioides Lophonetta Brazilian Teal, Amazonetta brasiliensis Amazonetta Spectacled Duck / Bronze-winged Duck, Speculanas specularis Speculanas Falkland Steamer-Duck, Tachyeres brachypterus Flightless Steamer-Duck / Fuegian Steamer-Duck, Tachyeres pteneres Tachyeres Flying Steamer-Duck, Tachyeres patachonicus White-headed Steamer-Duck / Chubut Steamer-Duck, Tachyeres leucocephalus Baikal Teal, Sibirionetta formosa Sibirionetta Garganey, Spatula querquedula Hottentot Teal, Spatula hottentota Puna Teal, Spatula puna Silver Teal, Spatula versicolor Spatula Red Shoveler, Spatula platalea Blue-winged Teal, Spatula discors Cinnamon Teal, Spatula cyanoptera Cape Shoveler, Spatula smithii Northern Shoveler, Spatula clypeata Australasian Shoveler, Spatula rhynchotis Gadwall, Anas strepera Falcated Duck, Anas falcata Eurasian Wigeon, Anas penelope (Mareca) ?Amsterdam Wigeon, Anas marecula American Wigeon, Anas americana Chiloe Wigeon, Anas sibilatrix African Black Duck, Anas sparsa Yellow-billed Duck, Anas undulata Meller’s Duck, Anas melleri (Anas) Philippine Duck, Anas luzonica Anas Pacific Black Duck, Anas superciliosa Laysan Duck, Anas laysanensis Hawaiian Duck, Anas wyvilliana Indian Spot-billed Duck, Anas poecilorhyncha Mallard, Anas platyrhynchos Eastern Spot-billed Duck, Anas zonorhyncha American Black Duck, Anas rubripes Mexican Duck, Anas diazi Mottled Duck, Anas fulvigula Eurasian Teal, Anas crecca Green-winged Teal, Anas carolinensis (Dafilonettion) ?Andean Teal, Anas andium Yellow-billed -

Birds at Woodland Park Zoo Pre-Visit Information for Teachers
BIRDS AT WOODLAND PARK ZOO PRE-VISIT INFORMATION FOR TEACHERS If you are planning a zoo field trip and wish to have your students focus on birds during their visit, this pre-visit sheet can help them get the most out of their time at the zoo. We have put together an overview of key concepts related to birds, a list of basic vocabulary words, and a checklist of bird species at Woodland Park Zoo. Knowledge and understanding of these main ideas will enhance your students’ zoo visit. OVERVIEW: There are over 10,000 species of birds currently identified worldwide, inhabiting a number of different biomes and exhibiting a range of adaptations. Woodland Park Zoo exhibits a wide variety of bird species (see attached checklist) in several different areas of the zoo. A bird field trip to the zoo could focus on the characteristics of birds (see “Concepts” below), comparing/contrasting different birds or learning about biomes and observing the physical characteristics of birds in different biomes. CONCEPTS: Birds share the following physical characteristics: Feathers Endothermic (warm-blooded) Eggs with shell and yolk Lack teeth, but have bony beaks Lightweight skeleton, bones with air spaces Good vision Adaptations for flight: Low body weight Streamlined form Efficient metabolism Specialized respiration and circulation Birds, like all plants and animals, have five basic needs to survive—food, water, shelter, air and space. They inhabit every continent on the planet and range in size from the bee hummingbird at 0.05 ounces (1.6 grams) to the North African ostrich at 275 pounds (125 kilograms). -

Respiratory Mechanics of High Altitude Waterfowl
RESPIRATORY MECHANICS OF HIGH ALTITUDE WATERFOWL by Julia McRae York B.Sc., The University of British Columbia 2015 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in THE FACULTY OF GRADUATE AND POSTDOCTORAL STUDIES (Zoology) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) August, 2016 © Julia McRae York, 2016 ABSTRACT Birds living at high altitude (>3,000 meters) are not only able to cope with reduced oxygen availability due to hypobaria, but they are also able to achieve one of the most metabolically costly form of locomotion at these altitudes: flight. To perform such a metabolically demanding activity, in addition to energetically expensive daily tasks such as foraging, predator escape, and reproduction, all in oxygen limited (hypoxic) conditions, means that high altitude birds must enhance oxygen supply to maintain oxygen homeostasis. The primary means of increasing oxygen supply is increasing ventilation of the respiratory gas exchange surface. However, the metabolic cost of ventilation is unknown for birds at rest, as is whether this cost varies among bird species. In this thesis, the cost and work of breathing are compared in fourteen avian species to determine whether variation in the work of breathing is due to mechanical or morphological changes in the respiratory system, and if any observed changes are associated with living at high altitude. High altitude birds tended to have large and compliant respiratory systems compared with low altitude taxa, which reduces the work of breathing. However, the evidence also suggests that respiratory morphology and mechanics in birds may be more constrained by life history strategy than by evolutionary time at altitude, although species in this study that have no high altitude sister taxa (their lineages have never radiated to high altitude) struggled the most with increasing oxygen supply. -

2020 HOAGBA Directory Listing.Pdf
Listing for the HOAGBA Membership Directory Name___________________________________ Farm Name_____________________________Ph. #______________________ Address________________________________________City, State & Zip +4___________________________________________ E-mail __________________________________________Web Site: _________________________________________________ You will not be listed in the directory unless this list is completed and returned to Terry Smith, 14000 W. 215th St., Bucyrus, KS 66013 by June 30, 2020. Complete the form, by putting a check mark in front of each species that you currently own. If you have species not listed, list them on a separate sheet of paper & staple it to this one. Please print legibly or type the that information. Australian Greater Magellan Gambel's True Pheasants Tragopans New Zealand Cereopsis Chestnut Bellied Ringneck Satyr Radjah Blue Scaled White Temminck’s Swans Common Elegant (Benson) Jumbo Ringneck Cabots Mute Mountain Blackneck Pochards Polish Others Mearns Bianchi Red Crested Black Malay Argus Button Green Mute Rosybill Black-neck Impeyan Versicolor Canvasback Trumpeter Grouse Koklass Mongolian Redhead Coscoraba Ruffed Cheer Buff Common Whiteye Whooper Sharptail Other Ringnecks - List Dabbling Ducks Ring-Neck Whistler Blue Marbled Teal Tufted Prairie Chicken Ruffed Peafowl Cape Teal Lesser Scaup Spruce Lady Amherst India Blue Hottentot Teal Greater Scaup Golden (Red) Black Shoulder Jungle Fowl Northern Silver Teal Ghigi (Yellow) Golden Perching Ducks White Red Southern Silver Teal Dark-Throated Lesser Brazilian Teal Spalding Sonnerats Puna Teal Silver Golden Gr. Brazilian Teal Pied Ceylon Lesser Bahama Teal Salmon Golden Maned Goose Green Green Greater Bahama Teal Cinammon Golden Mandarin Cameo Northern Pintail Tinamou Other Goldens - List Wood Duck Oaten Chilean Teal Crested White-Winged Wood D. Other colors Gallo Sharpwing Teal Chilean Barrows Goldeneye Nepal Kalij Am. -

BIRDS of BOLIVIA UPDATED SPECIES LIST (Version 03 June 2020) Compiled By: Sebastian K
BIRDS OF BOLIVIA UPDATED SPECIES LIST (Version 03 June 2020) https://birdsofbolivia.org/ Compiled by: Sebastian K. Herzog, Scientific Director, Asociación Armonía ([email protected]) Status codes: R = residents known/expected to breed in Bolivia (includes partial migrants); (e) = endemic; NB = migrants not known or expected to breed in Bolivia; V = vagrants; H = hypothetical (observations not supported by tangible evidence); EX = extinct/extirpated; IN = introduced SACC = South American Classification Committee (http://www.museum.lsu.edu/~Remsen/SACCBaseline.htm) Background shading = Scientific and English names that have changed since Birds of Bolivia (2016, 2019) publication and thus differ from names used in the field guide BoB Synonyms, alternative common names, taxonomic ORDER / FAMILY # Status Scientific name SACC English name SACC plate # comments, and other notes RHEIFORMES RHEIDAE 1 R 5 Rhea americana Greater Rhea 2 R 5 Rhea pennata Lesser Rhea Rhea tarapacensis , Puna Rhea (BirdLife International) TINAMIFORMES TINAMIDAE 3 R 1 Nothocercus nigrocapillus Hooded Tinamou 4 R 1 Tinamus tao Gray Tinamou 5 H, R 1 Tinamus osgoodi Black Tinamou 6 R 1 Tinamus major Great Tinamou 7 R 1 Tinamus guttatus White-throated Tinamou 8 R 1 Crypturellus cinereus Cinereous Tinamou 9 R 2 Crypturellus soui Little Tinamou 10 R 2 Crypturellus obsoletus Brown Tinamou 11 R 1 Crypturellus undulatus Undulated Tinamou 12 R 2 Crypturellus strigulosus Brazilian Tinamou 13 R 1 Crypturellus atrocapillus Black-capped Tinamou 14 R 2 Crypturellus variegatus -

Mobility, Subsistence, and Technological Strategies of Early Holocene Hunter-Gatherers in the Bolivian Altiplano
Quaternary International xxx (2017) 1e16 Contents lists available at ScienceDirect Quaternary International journal homepage: www.elsevier.com/locate/quaint Mobility, subsistence, and technological strategies of early Holocene hunter-gatherers in the Bolivian Altiplano * Jose M. Capriles a, , Juan Albarracin-Jordan b, Douglas W. Bird a, Steven T. Goldstein c, Gabriela M. Jarpa d, Sergio Calla Maldonado e, Calogero M. Santoro f a Department of Anthropology, The Pennsylvania State University, University Park, PA 16802, USA b Instituto de Investigaciones Antropologicas y Arqueologicas, Universidad Mayor de San Andres, La Paz, Bolivia c Department of Archaeology, Max Planck Institute for the Science of Human History, Jena, Germany d Departamento de Antropología, Universidad de Tarapaca, Arica, Chile e Carrera de Arqueología, Universidad Mayor de San Andres, La Paz, Bolivia f Instituto de Alta Investigacion, Universidad de Tarapaca, Arica, Chile article info abstract Article history: The Altiplano constitutes the most extensive, high-elevation terrain in South America. Most archaeo- Received 15 January 2017 logical research on the earliest human occupation of this region in the Bolivian Andes derives from sites Received in revised form such as Viscachani where the emphasis has been on typological comparisons of projectile points, rather 20 August 2017 than on complete and radiometrically dated assemblages. In this paper, we present survey and exca- Accepted 30 August 2017 vation data from the Iroco region in the Central Altiplano of Bolivia to address questions related to the Available online xxx adaptive strategies engaged by Archaic Period highland foragers. Specifically, we focus on the nature of mobility, subsistence, and technological strategies, stemmed from principles in human behavioral Keywords: Andean Altiplano ecology.