Integrated Approach to the Control of Lymphatic Filariasis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Liberia Environmental Profile
Liberia environmental profile This report is financed by the European commission and is presented by Agreco G.E.I.E for the Government of Liberia and the European Commission. It does not necessarily reflect the opinion of the Government or the European Commission. Authors: Dr Giorgio V. Brandolini, agronomist, biodiversity expert Dr Mohammed Tigani, environmental policy and management expert (industry, water, and waste management) Monrovia, December 2006 1 List of abbreviations and acronims Acronims GoL Government of Liberia iPRS interim Poverty Reduction Strategy PRSP Poverty reduction strategy paper DDRR Disarmament, Demobilisation, Reinsertion and Reintegration NIP EDF 9 National Indicative Programme 9th EDF 9th European development fund CSP Country Strategy Paper NIP National Indicative Programme 10th EDF 10th European development fund EPA Environmental Protection Agency EIA Environmental Impact Assessment MEAs Multilateral Environmental Agreements CEP Country Environmental Profile MDG 7 Millenium development goals 7 FLEGT Eu action plan for Forest Law Enforcement, Governance and Trade 2 Table of contents 1. Summary..................................................................................................................................... 5 2. Introduction............................................................................................................................... 10 3. State of the environment ........................................................................................................... 11 3.1 -

Subproject Briefs
Liberia Energy Sector Support Program (LESSP) Subproject Briefs 8 July 2013 LESSP Subprojects Introduction • Seven Infrastructure Subprojects – OBJECTIVE 2 – Pilot RE Subprojects • Two hydro (one Micro [15 kW] and one Mini [1,000 kW]) • Two biomass power generation – OBJECTIVE 3 – Support to Liberia Energy Corporation (LEC) • 1000 kW Photovoltaic Power Station interconnected to LEC’s grid • 15 km Electric Distribution Line Extension to University of Liberia (UL) Fendell Campus – OBJECTIVE 3 - Grants – Public Private Partnership • One Biomass Power Generation Research and Demonstration (70 kW) • Total Cost: $ 13.97 Million USD (Engineer’s Estimate) • Service to: More than an estimated 72,000 Liberians (3,600 households and over 160 businesses and institutions) Subprojects Summary Data Project Cost, Service No LESSP Subprojects County kW Beneficiaries USD Population Million Mein River Mini Hydropower Subproject Bong 7.25 Over 3000 households, 150 1 1,000 Over 25,000 businesses and institutions Wayavah Falls Micro Hydropower Subproject Lofa 0.45 150 households and 4-5 2 15 Over 1,000 businesses/institutions Kwendin Biomass Electricity Subproject Nimba 0.487 248 households, a clinic, and a 3 60 Over 2,000 school Sorlumba Biomass Electricity Subproject Lofa 0.24 206 households, 8 institutions 4 35 Over 1,500 and businesses Grid connected 1 MW Solar PV Subproject Montserrado 3.95 5 1,000 LEC grid Over 15,000 MV Distribution Line Extension to Fendell Montserrado 1.12 6 Fendell Campus Over 25,000 Campus Establishment of the Liberia Center for Biomass Margibi 0.467 7 70 BWI Campus, RREA Over 2,200 Energy at BWI TOTAL - 5 counties 13.97 2,161 3,600 households and over 160 Over 72,000 businesses and institutions Liberia Energy Sector Support Program Subproject Brief: Mein River 1 MW Mini-Hydropower Subproject Location Suakoko District, Bong County (7o 8’ 11”N 9o 38’ 27” W) General Site The power house is 3 km uphill from the nearest road, outside the eco- Description tourism area of the Lower Kpatawee Falls. -

Congressional Budget Justification 2015
U.S. AFRICAN DEVELOPMENT FOUNDATION Pathways to Prosperity “Making Africa’s Growth Story Real in Grassroots Communities” CONGRESSIONAL BUDGET JUSTIFICATION Fiscal Year 2015 March 31, 2014 Washington, D.C. United States African Development Foundation (This page was intentionally left blank) 2 USADF 2015 CONGRESSIONAL BUDGET JUSTIFICATION United States African Development Foundation THE BOARD OF DIRECTORS AND THE PRESIDENT OF THE UNITED STATES AFRICAN DEVELOPMENT FOUNDATION WASHINGTON, DC We are pleased to present to the Congress the Administration’s FY 2015 budget justification for the United States African Development Foundation (USADF). The FY 2015 request of $24 million will provide resources to establish new grants in 15 African countries and to support an active portfolio of 350 grants to producer groups engaged in community-based enterprises. USADF is a Federally-funded, public corporation promoting economic development among marginalized populations in Sub-Saharan Africa. USADF impacts 1,500,000 people each year in underserved communities across Africa. Its innovative direct grants program (less than $250,000 per grant) supports sustainable African-originated business solutions that improve food security, generate jobs, and increase family incomes. In addition to making an economic impact in rural populations, USADF’s programs are at the forefront of creating a network of in-country technical service providers with local expertise critical to advancing Africa’s long-term development needs. USADF furthers U.S. priorities by directing small amounts of development resources to disenfranchised groups in hard to reach, sensitive regions across Africa. USADF ensures that critical U.S. development initiatives such as Ending Extreme Poverty, Feed the Future, Power Africa, and the Young African Leaders Initiative reach out to those communities often left out of Africa’s growth story. -

Determinants of the Economic Efficiency of Cassava Production in Bomi and Nimba Counties, Liberia”____
DETERMINANTS OF THE ECONOMIC EFFICIENCY OF CASSAVA PRODUCTION IN BOMI AND NIMBA COUNTIES, LIBERIA KOLLIE B. DOGBA A56/9511/2017 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE AWARD OF THE DEGREE OF MASTER OF SCIENCE IN AGRICULTURAL AND APPLIED ECONOMICS DEPARTMENT OF AGRICULTURAL ECONOMICS FACULTY OF AGRICULTURE UNIVERSITY OF NAIROBI 2020 DECLARATION This thesis is my original work that has not been presented for award of a degree in any other University. This thesis has been submitted with our approval as University supervisors: ii DECLARATION OF ORIGINALITY University of Nairobi This form must be completed and signed for all works submitted to the University for Examination Name of Student: KOLLIE B. DOGBA_______________________________ Registration Number: A56/9511/2017________________________________ College: COLLEGE OF AGRICULTURE & VETERINARY SCIENCES (CAVS) Faculty/School/Institute: FACULTY OF AGRICULTURE___________________________ Department: AGRICULTURAL ECONOMICS__________________________ Course Name: Agricultural and Applied Economics (MSc. Program)________ Title of the Work: “DETERMINANTS OF THE ECONOMIC EFFICIENCY OF CASSAVA PRODUCTION IN BOMI AND NIMBA COUNTIES, LIBERIA”____ DECLARATION 1. I understand what plagiarism is and I am aware of the University’s policy in this regard 2. I declare that this ____THESIS__ (Thesis, project, essay, assignment, paper, report, etc) is my original work and has not been submitted elsewhere for examination, award of a degree or publication. Where other people’s work or my own work has been used, this has properly been acknowledged and referenced in accordance with the University of Nairobi’s requirements. 3. I have not sought or used the services of any professional agencies to produce this work 4. -

First State of the Environment Report for Liberia - 2006 First State of the Environment Report for Liberia - 2006
FIRST STATE OF THE ENVIRONMENT REPORT FOR LIBERIA - 2006 FIRST STATE OF THE ENVIRONMENT REPORT FOR LIBERIA - 2006 Copyright © 2006 United Nations Development Programme This report has been prepared by the United Nations Development Programme for the Government of Liberia. Reproduction of this publication for educational or any other non- commercial purpose is authorized without prior permission from the copyright holder, provided the source is acknowledged. Reproduction for resale or other purposes is prohibited without the prior permission in writing from the Government. Produced by UNDP P.O. Box 274 Monrovia, Liberia Vsat: 0031205407121 or 122 Fax: +31205407127 E-M: [email protected] In Collaboration with the Environmental Protection Agency (EPA) of Liberia P.O. Box 4024 4th Street, Tubman Blvd., Sinkor 1000 Monrovia 10, Liberia ii Table of contents FOREWORD...........................................................................................................................................................vi Acknowledgement..............................................................................................................................................viii Executive Summary .............................................................................................................................................ix 1 OVERVIEW OF LIBERIA’S ENVIRONMENT ...........................................................................................11 Introduction ..............................................................................................................................................................11 -

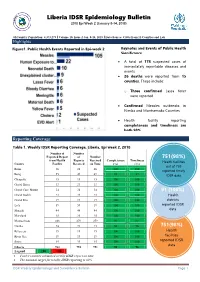
Liberia IDSR Epidemiology Bulletin 2017 Epi Week 38 (Sept
Liberia IDSR Epidemiology Bulletin 2017 Epi Week 38 (Sept. 18-24, 2017) Country Population: 4,373,279 l Volume 09, Issue 38 Sept. 18 – 24, 2017 l Data Source: CSOs from 15 Counties and Lab Highlights Figure 1. Public Health Events Reported in Epi- Keynotes and Events of Public Health week 38 Significance n A total of ninety-one suspected cases of immediately reportable diseases and events including 23 deaths were reported from 15 counties Health facility reporting completeness and timeliness are 98% and 94% respectively Measles outbreaks confirmed in Bong and Nimba Counties Eight neonatal deaths identified retrospectively in Bong County Grand Kru County reached alert threshold for meningitis with six suspected cases reported. o However, only one case was confirmed and the other five discarded due to negative lab results Reporting Coverage Table 1. Weekly IDSR Reporting Coverage, Liberia, Epi week 38, 2017 Number of Number of Number Expected Health Reports Received Completeness Timeliness 716 (94%) County Facility Report Received on Time (%) (%) Health facilities Bomi 23 23 0 100 0 out of 759 Bong 55 54 54 98 98 reported timely Gbarpolu 15 15 15 100 100 IDSR data Grand Bassa 33 33 33 100 100 Grand Cape Mount 32 32 32 100 100 Grand Gedeh 24 24 24 100 100 Grand Kru 19 19 19 100 100 91 (100%) Lofa 59 59 59 100 100 Health districts Margibi 44 44 44 100 100 reported IDSR Maryland 25 25 25 100 100 data Montserrado 283 283 283 100 100 Nimba 74 74 74 100 100 Rivercess 19 19 19 100 100 758(98%) River Gee 19 19 0 100 0 Health facilities Sinoe 35 35 35 100 100 reported IDSR Liberia (National) 759 758 716 97 94 data Legend ≥80 <80 All counties submitted weekly IDSR report on time except River Gee & Bomi counties The national target for weekly IDSR reporting is 80% IDSR Weekly Epidemiology and Surveillance Bulletin Page 1 Liberia IDSR Epidemiology Bulletin 2017 Epi Week 38 (Sept. -

Lofa County Development Agenda Republic of Liberia Lofa County Development Agenda 2008 – 2012 County Vision Statement
Lofa County Development Agenda Republic of Liberia Lofa County Development Agenda 2008 – 2012 County Vision Statement Lofa County shall be a united, secure center of excellence in the delivery of social and infrastructure services and poverty reduction for all. Core Values Equal access to opportunities for all Lofa citizens Restoration of peace, security and the rule of law Transparent and effective governance economic growth and job creation Preservation of natural resources and environmental protection Republic of Liberia Prepared by the County Development Committee, in collaboration with the Ministries of Planning and Economic Affairs and Internal Affairs. Supported by the UN County Support Team project, funded by the Swedish Government and UNDP. Table of Contents A MESSAGE FROM THE MINISTER OF INTERNAL AFFAIRS........! iii FOREWORD..........................................................................! iv PREFACE!!............................................................................. vi LOFA COUNTY OFFICIALS....................................................! viii EXECUTIVE SUMMARY..........................................................! xi PART 1 - INTRODUCTION AND BACKGROUND 1.1.!Introduction................................................................................................! 1 1.2.!History........................................................................................................! 1 1.3.!Geography..................................................................................................! -

Advancing Youth Project: Labor Market Assessment Report
Advancing Youth Project Labor Market Assessment — Liberia This report is made possible by the generous support of the American people through the United States Agency for International Development, USAID/Liberia Cooperative Agreement No. 669-A-11-00001 to Education Development Center. The content and opinions expressed within do not necessarily reflect the views of USAID or the United States Government. Advancing Youth Project: Labor Market Assessment i Acknowledgements The Labor Market Assessment (LMA) team hereby conveys thanks to the Government of Liberia and the Ministry of Education for the establishment of Alternative Basic Education program targeting unschooled youth. We would also like to express our appreciation to the United States Agency for International Development (USAID) for funding the Advancing Youth project. Special thanks to Mrs. Mardea Nyumah, of USAID in Monrovia for providing technical guidance and advice throughout the design and review process of the LMA. We are also grateful to Mr. S. Tornorlah Varpilah, the Minister of Youth and Sports for his contribution of time and information which formed an important part of this report. To the many traders and companies who were interviewed during the survey, farmers and traders who participated in value chain analysis and key informants from private sector, NGOs and government institutions, to all we owe you many thanks. The USAID/Advancing Youth team including partner organizations, Education Development Center (EDC), Mercy Corps and YMCA led by Chief of Party, Simon James, was instrumental in providing valuable administrative support and team coordination. To all team coordinators, thanks for the tireless effort. YMCA played a crucial role by providing competent youth assessors, who worked hard to design data collection tools, administered the assessment in the five counties and finalized data entry process smoothly. -
Liberia IDSR Epidemiology Bulletin
ATION N AL P U B L I C Liberia IDSR Epidemiology Bulletin H E A A L I T R 2018 Epi Week 2 (January 8-14, 2018) H E B I I N L S T F I O T U E T 01Country Population: 4,373,279 l Volume 10, Issue 2 Jan. 8-14, 2018 l Data Source: CSOs from 15 Counties and Lab Highlights Keynotes and Events of Public Health Figure 1. Public Health Events Reported in Epi-week 2 Significance A total of 178 suspected cases of immediately reportable diseases and events 26 deaths were reported from 15 counties. These include: o Three confirmed Lassa fever were reported Confirmed Measles outbreaks in Nimba and Montserrado Counties Health facility reporting completeness and timeliness are both 98% Reporting Coverage Table 1. Weekly IDSR Reporting Coverage, Liberia, Epi week 2, 2018 Number of Number Expected Report of Number 751(98%) from Health Reports Received Completeness Timeliness Health facilities County Facility Received on Time (%) (%) out of 759 Bomi 26 26 26 100 100 reported timely Bong 55 49 49 89 89 IDSR data Gbarpolu 15 15 15 100 100 Grand Bassa 33 33 33 100 100 Grand Cape Mount 34 34 34 100 100 91 (100%) Grand Gedeh 24 24 24 100 100 Health Grand Kru 19 19 19 100 100 districts reported IDSR Lofa 59 59 59 100 100 Margibi 44 44 44 100 100 data Maryland 25 25 25 100 100 Montserrado 283 279 279 99 99 Nimba 74 71 71 96 96 751(98%) Health Rivercess 19 19 19 100 100 River Gee 19 19 19 100 100 facilities Sinoe 35 35 35 100 100 reported IDSR data Liberia 764 751 751 98 98 Legend ≥80 <80 Twelve counties submitted weekly IDSR report on time The national target for weekly IDSR reporting is 80% IDSR Weekly Epidemiology and Surveillance Bulletin Page 1 ATION N AL P U B L I C Liberia IDSR Epidemiology Bulletin H E A A L I T R 2018 Epi Week 2 (January 8-14, 2018) H E B I I N L S T F I O T U E T Vaccine Preventable Diseases Measles One Hundred and Five (105) suspected cases were reported from Montserrado (45), Nimba (40), Grand Bassa (4) Bong (4), Margibi (3), Grand Kru (2), Grand Gedeh (2), Maryland (2), Bomi (1), Lofa (1) and Sinoe (1) Counties. -

Congressional Budget Justification Fiscal Year 2017
U.S. AFRICAN DEVELOPMENT FOUNDATION “Creating Pathways to Prosperity” CONGRESSIONAL BUDGET JUSTIFICATION Fiscal Year 2017 The U.S. African Development Foundation (USADF) is an independent agency of the U.S. federal government, funding grassroots development projects to African-owned and led enterprises, cooperatives and community-based organizations. Our objective is to build African communities’ capacity, resilience, and economic activities at the community level so all Africans can contribute to Africa’s growth story. USADF is on the frontier of development, working directly with Africans on the ground to combat some of Africa’s most difficult development challenges with programs to increase U.S. development presence in the hardest to reach areas of extreme poverty. USADF grants (up to $250,000 each), enable our grantees to address the root causes of poverty, hunger, and lack of infrastructure (particularly energy poverty) in their communities to: Combat hunger through resilience, agricultural, and livestock programming Improve access to local and regional markets for small-holder farmers, cooperatives and entrepreneurs Empower women and girls to create and control their own economic livelihoods Create job opportunities and resources for youth through training Promote African solutions to the lack of basic infrastructure, particularly in rural areas and urban slums March 3, 2016 Washington, D.C. www.USADF.gov (This page was intentionally left blank) www.USADF.gov United States African Development Foundation THE BOARD OF DIRECTORS AND THE PRESIDENT OF THE UNITED STATES AFRICAN DEVELOPMENT FOUNDATION WASHINGTON, DC We are pleased to present the Administration’s FY 2017 budget justification for the United States African Development Foundation (USADF). -

QUARTERLY REPORT July to September 2013 Submitted to USAID/Liberia
CSML Civil Society and Media Leadership Program QUARTERLY REPORT July to September 2013 Submitted to USAID/Liberia Award Number: 669-A-00-10-00074-00 Grantee: IREX Contacts: IREX Washington IREX Liberia Jill Miller Bill Burke 1275 K Street, NW Payne Avenue & 15th Street Suite 600 Sinkor, Monrovia Washington, DC 20005 Liberia U.S.A. 231(0)88-060-1859 1 202-628-8188 TABLE OF CONTENTS 1. EXECUTIVE SUMMARY ............................................................................................................................................ 5 2. PROGRAM PURPOSE ................................................................................................................................................ 6 3. PROGRAM RESULTS ................................................................................................................................................. 6 4. PROGRAM ACTIVITIES ............................................................................................................................................ 8 4.1. Management Overview ................................................................................................................................... 8 4.2. Finance, Administration and IT ...................................................................................................................... 8 4.2.1. Finance ..................................................................................................................................................... 8 4.2.2. Administration ........................................................................................................................................ -

Note to Ms. Alicia Barcena UNITED NATIONS TRUST FUND FOR
Note to Ms. Alicia Barcena UNITED NATIONS TRUST FUND FOR HUMAN SECURITY I have reviewed the attached project proposal from the Liberia inter-agency project team (UNDP, WFP, FAO) entitled, "Rebuilding communities in post-conflict Liberia - empowerment for change". This project requiring $3,857,867.66 is in line with the guidelines governing the United Nations Trust Fund for Human Security and has been endorsed by the Project Review Committee. I hereby request your approval of this project. tf MAY 1 5 2006 PROGRAMME DOCUMENT National Transitional Government of Liberia United Nations Development Programme Food and Agriculture Organization of the United Nations World Food Programme Rebuilding Communities in Post-Conflict Liberia - Empowerment for Change Project Number: Project Title: Rebuilding Communities in Post-Conflict Liberia Project Site: Lofa, Bomi, Bong, Maryland, Nimba, Gbapolu, Grand Cape, Grand Geddeh, & River Gee Counties. Estimated start date: Estimated end date: Duration: 2 Years Project Execution: UNDP, FAO, and WFP Counterpart Institutions: Local Governments, NGOs & District Development Committees -DDCs Estimated budget: US$ 3,857,867.66: (Phase I = $ 1,981,443.73 and Phase II = $1,876,423.93) Sector: Employment, Social Infrastructure, Agriculture and Reconciliation Programme Brief: Poverty and unemployment were the main contributors to the decade old protracted and violent conflict in Liberia. Provision of basic services like education and health care, and the creation of opportunities for productive and gainful employment are interventions that are expected to positively motivate communities to support the consolidation of peace. The Programme will be expected to lower social tensions and contribute to the creation of a safe and secure environment for local communities enabling them to recognize and benefit from the peace dividend, and constructively participate in the recovery towards peace.