Ecology of the River Cooter, Pseudemys Concinna, in a Southern Illinois Floodplain Lake
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Year of the Turtle News No
Year of the Turtle News No. 1 January 2011 Basking in the Wonder of Turtles www.YearoftheTurtle.org Welcome to 2011, the Wood Turtle, J.D. Kleopfer Bog Turtle, J.D. Willson Year of the Turtle! Turtle conservation groups in partnership with PARC have designated 2011 as the Year of the Turtle. The Chinese calendar declares 2011 as the Year of the Rabbit, and we are all familiar with the story of the “Tortoise and the Hare”. Today, there Raising Awareness for Turtle State of the Turtle Conservation is in fact a race in progress—a race to extinction, and turtles, unfortunately, Trouble for Turtles Our Natural Heritage of Turtles are emerging in the lead, ahead The fossil record shows us that While turtles (which include of birds, mammals, and even turtles, as we know them today, have tortoises) occur in fresh water, salt amphibians. The majority of turtle been on our planet since the Triassic water, and on land, their shells make threats are human-caused, which also Period, over 220 million years ago. them some of the most distinctive means that we can work together to Although they have persisted through animals on Earth. Turtles are so address turtle conservation issues many tumultuous periods of Earth’s unique that some scientists argue that and to help ensure the continued history, from glaciations to continental they should be in their own Class of survival of these important animals. shifts, they are now at the top of the vertebrates, Chelonia, separate from Throughout the year we will be raising list of species disappearing from the reptiles (such as lizards and snakes) awareness of the issues surrounding planet: 47.6% of turtle species are and other four-legged creatures. -

In AR, FL, GA, IA, KY, LA, MO, OH, OK, SC, TN, and TX): Species in Red = Depleted to the Point They May Warrant Federal Endangered Species Act Listing
Southern and Midwestern Turtle Species Affected by Commercial Harvest (in AR, FL, GA, IA, KY, LA, MO, OH, OK, SC, TN, and TX): species in red = depleted to the point they may warrant federal Endangered Species Act listing Common snapping turtle (Chelydra serpentina) – AR, GA, IA, KY, MO, OH, OK, SC, TX Florida common snapping turtle (Chelydra serpentina osceola) - FL Southern painted turtle (Chrysemys dorsalis) – AR Western painted turtle (Chrysemys picta) – IA, MO, OH, OK Spotted turtle (Clemmys gutatta) - FL, GA, OH Florida chicken turtle (Deirochelys reticularia chrysea) – FL Western chicken turtle (Deirochelys reticularia miaria) – AR, FL, GA, KY, MO, OK, TN, TX Barbour’s map turtle (Graptemys barbouri) - FL, GA Cagle’s map turtle (Graptemys caglei) - TX Escambia map turtle (Graptemys ernsti) – FL Common map turtle (Graptemys geographica) – AR, GA, OH, OK Ouachita map turtle (Graptemys ouachitensis) – AR, GA, OH, OK, TX Sabine map turtle (Graptemys ouachitensis sabinensis) – TX False map turtle (Graptemys pseudogeographica) – MO, OK, TX Mississippi map turtle (Graptemys pseuogeographica kohnii) – AR, TX Alabama map turtle (Graptemys pulchra) – GA Texas map turtle (Graptemys versa) - TX Striped mud turtle (Kinosternon baurii) – FL, GA, SC Yellow mud turtle (Kinosternon flavescens) – OK, TX Common mud turtle (Kinosternon subrubrum) – AR, FL, GA, OK, TX Alligator snapping turtle (Macrochelys temminckii) – AR, FL, GA, LA, MO, TX Diamond-back terrapin (Malaclemys terrapin) – FL, GA, LA, SC, TX River cooter (Pseudemys concinna) – AR, FL, -

Caring for Your River Cooter Turtles
caring for your River Cooter Turtles Scientific Name: Pseudemys concinna Native to: Central and Eastern United States Maximum Length: Females up to 16 inches, males up to 10 inches Life Span: 40 + years characteristics: River Cooters are large turtles with relatively flat shells. River Cooters have a brown to black carapace, with reddish tinges, and the plastron is yellow, orange or reddish with prominent patterns of orange and black. Their head stripes are yellow, but may even seem orange. These gorgeous turtles are good for a beginner. River Cooters are typically found in large rivers with clear water, gravel river beds, and aquatic plants. care tips: Enclosure: Juvenile River Cooters can be kept in a 20 – 30 gallon long tank, adults require much larger accommodations. A minimum 300 gallon tank is needed to house an adult River Cooter. Substrate: Reptile sand or even fine pea gravel. Habitat: Cooters do well in aquariums when the water is kept clean and filtered. Make sure to provide plenty of space for your River Cooter including a basking area where they can get completely out of the water and swimming area with water deep enough to swim. Temperature and Lighting: Provide UVB lighting, a basking area of 85 degrees and water temperature of 75 degrees are recommended for these turtles. The basking platform must allow River Cooters enough room to stretch out and fully dry their shell and plastron to avoid shell rot. Food and Water: River Cooters are omnivores. Their diet should consist of a mix of pelleted turtle food, crickets, mealworms, and leafy greens such as romaine, collard, and turnip greens.. -

What's Inside
What’s Inside 1 Greeting 2 Project Updates 5 In Action 7 Field Techniques 14 Season Highlights 15 The PARS Experience 20 Meet the Volunteers 21 Species Spotlight 27 Wanted Species Photo: Brandon Hunsberger A Partnership Project of The Mid-Atlantic Center for Herpetology and Conservation and The Pennsylvania Fish & Boat Commission 1 Greetings SpringSpring 20162016 Once again, it was a genuine pleasure to see so many of our volunteers in the same room as we held our second Annual PARS Meeting on the cusp of this year’s vernal season. Good vibes permeated the atmosphere with ample opportunity for members to socialize. The guest speakers gave excellent presentations, the venue was beautiful and in an interesting location, and several new County Coordinators were recruited. One aspect of the meeting was the formal beginning of a partnership between PARS and Clarion University, with numerous participants trained to collect field samples for an important study designed to determine the presence distribution of amphibian diseases in Pennsylvania. This project is one of several ways PARS is partnering with other conservation efforts and a good example of how the PARS project reaches beyond simply documenting the presence of species. Other current examples include PARS volunteer participation in PA Fish & Boat projects focused on rare herp species, and in numerous bio-blitz events this year. Undoubtedly more partnerships will develop as our project continues. The annual meeting was a great kick-off to what is shaping up to be another great year for PARS. Volunteer recruitment also continues at an impressive rate, and we have already received some incredible records this spring, including several new county records and a new block for the Eastern Smooth Earthsnake. -

The Natural History & Distribution of Riverine Turtles in West Virginia
Marshall University Marshall Digital Scholar Theses, Dissertations and Capstones 2010 The aN tural History & Distribution of Riverine Turtles in West Virginia Linh Diem Phu Follow this and additional works at: http://mds.marshall.edu/etd Part of the Aquaculture and Fisheries Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Phu, Linh Diem, "The aN tural History & Distribution of Riverine Turtles in West Virginia" (2010). Theses, Dissertations and Capstones. Paper 787. This Thesis is brought to you for free and open access by Marshall Digital Scholar. It has been accepted for inclusion in Theses, Dissertations and Capstones by an authorized administrator of Marshall Digital Scholar. For more information, please contact [email protected]. The Natural History & Distribution of Riverine Turtles in West Virginia Thesis submitted to the Graduate College of Marshall University In partial fulfillment of the requirements for the degree of Master of Science in Biological Sciences By Linh Diem Phu Dr. Thomas K. Pauley, Ph.D., Committee Chairperson Dr. Dan Evans, Ph.D. Dr. Suzanne Strait, Ph.D. Marshall University May 2010 Abstract Turtles are unique evolutionary marvels that evolved from amphibians and developed their protective shelled form more than 200 million years ago. In West Virginia, there are 10 native species of turtles, 9 of which are aquatic. Most of these aquatic turtles feed on carrion and dead plant matter, in the water and essentially "clean" our water systems. Turtles are long-lived animals with sensitive life stages that can serve as both long-term and short-term bioindicators of environmental health. With the increase in commercial trade, habitat fragmentation, degradation, destruction, there has been a marked decline in turtle species. -

Turtles of the Upper Mississippi River System
TURTLES OF THE UPPER MISSISSIPPI RIVER SYSTEM Tom R. Johnson and Jeffrey T. Briggler Herpetologists Missouri Department of Conservation Jefferson City, MO March 27, 2012 Background: A total of 13 species and subspecies of turtles are known to live in the Upper Mississippi River, its backwaters and tributaries. There are a few species that could be found occasionally, but would likely account for less than 5% of the species composition of any area. These species are predominantly marsh animals and are discussed in a separate section of this paper. For additional information on turtle identification and natural history see Briggler and Johnson (2006), Christiansen and Bailey (1988), Conant and Collins (1998), Ernst and Lovich (2009), Johnson (2000), and Vogt (1981). This information is provided to the fisheries field staff of the LTRM project so they will be able to identify the turtles captured during fish monitoring. The most current taxonomic information of turtles was used to compile this material. The taxonomy followed in this publication is the Scientific and Standard English Names of Amphibians and Reptiles of North America North of Mexico, with comments regarding confidence in our understanding (6th edition) by Crothers (2008). Species Identification, Natural History and Distribution: What follows is a synopsis of the 13 turtle species and subspecies which are known to occur in the Upper Mississippi River environs. Species composition changes between the upper and lower reaches of the LTRM study area (Wisconsin/Minnesota state line and southeastern Missouri) due to changes in aquatic habitats. For example, the Northern Map Turtle (Graptemys geographica) is abundant in the northern portion of the river with clearer water and abundant snail prey. -

Site Selection and Survival of Pseudemys Texana and Trachemys
SITE SELECTION AND SURVIVAL OF PSEUDEMYS TEXANA AND TRACHEMYS SCRIPTA ELEGANS NESTS AT SPRING LAKE IN SAN MARCOS, TEXAS THESIS Presented to the Graduate Council of Texas State University-San Marcos in Partial Fulfillment of the Requirements for the Degree Master of SCIENCE by Alycia Catherine Washington, B.S San Marcos, Texas August, 2008 SITE SELECTION AND SURVIVAL OF PSEUDEMYS TEXANA AND TRACHEMYS SCRIPTA ELEGANS NESTS AT SPRING LAKE IN SAN MARCOS, TEXAS Committee Members Approved: _______________________________ Thomas R. Simpson, Chair _______________________________ Francis Rose _______________________________ Clay Green Approved: ______________________________ J. Michael Willoughby Dean of Graduate College Dedication To my grandmothers, Catherine and Inez. ACKNOWLEDGMENTS I would like to give special thanks to everyone who contributed to the completion of this project and the quality of my experience while pursuing my graduate degree. Special thanks to my committee: To Dr. Randy Simpson, thank you for training my mind to think as a wildlife biologist. And for being exactly what I needed in an advisor- an excellent teacher and a great motivator despite my hesitations. Dr. Francis Rose, for sharing your knowledge of the turtles of Spring Lake with me. Your ideas were invaluable. To Dr. Clay Green, for always being open and willing to talk to me, especially when it had nothing to do with my research. Thank you also to Dr. Butch Weckerly, our friendly neighborhood statistician. Thank you for your willingness to answer my questions, even when they came in the hallway in passing. To Dr. David Lemke, for hiring me as an instructional assistant. To Hardin Rahe, thanks for being a strong influence since I was an undergrad and always pushing me to go further. -

A Systematic Review of the Turtle Family Emydidae
67 (1): 1 – 122 © Senckenberg Gesellschaft für Naturforschung, 2017. 30.6.2017 A Systematic Review of the Turtle Family Emydidae Michael E. Seidel1 & Carl H. Ernst 2 1 4430 Richmond Park Drive East, Jacksonville, FL, 32224, USA and Department of Biological Sciences, Marshall University, Huntington, WV, USA; [email protected] — 2 Division of Amphibians and Reptiles, mrc 162, Smithsonian Institution, P.O. Box 37012, Washington, D.C. 200137012, USA; [email protected] Accepted 19.ix.2016. Published online at www.senckenberg.de / vertebrate-zoology on 27.vi.2016. Abstract Family Emydidae is a large and diverse group of turtles comprised of 50 – 60 extant species. After a long history of taxonomic revision, the family is presently recognized as a monophyletic group defined by unique skeletal and molecular character states. Emydids are believed to have originated in the Eocene, 42 – 56 million years ago. They are mostly native to North America, but one genus, Trachemys, occurs in South America and a second, Emys, ranges over parts of Europe, western Asia, and northern Africa. Some of the species are threatened and their future survival depends in part on understanding their systematic relationships and habitat requirements. The present treatise provides a synthesis and update of studies which define diversity and classification of the Emydidae. A review of family nomenclature indicates that RAFINESQUE, 1815 should be credited for the family name Emydidae. Early taxonomic studies of these turtles were based primarily on morphological data, including some fossil material. More recent work has relied heavily on phylogenetic analyses using molecular data, mostly DNA. The bulk of current evidence supports two major lineages: the subfamily Emydinae which has mostly semi-terrestrial forms ( genera Actinemys, Clemmys, Emydoidea, Emys, Glyptemys, Terrapene) and the more aquatic subfamily Deirochelyinae ( genera Chrysemys, Deirochelys, Graptemys, Malaclemys, Pseudemys, Trachemys). -

Of Deadwood and Map Turtles (Graptemys): on Long Pond and Big Lake, Respectively, and to T .L
LINNAEUS FUND REsEARCH REPORTS 137 Assistantship Award. The majority of my thanks goes to E.O. Moll for his effort as a mentor, advisor, and editor, and Che Ionian Conservation and Biology. 1998. 3(1): I 37-14 I © 1998 by Chelonian Research Founda tion C.A. Phillips for his thorough review of this manuscript. I also thank E.L. Bryant amd J.R. Dreslik for their field assistance, E. Joyner and E. Bickett for allowing me to trap Of Deadwood and Map Turtles (Graptemys): on Long Pond and Big Lake, respectively, and to T .L. Esker An Analysis of Species Status for Five Species for help with White County sites. in Three River Drainages Using Replicated Spotting-Scope Counts of Basking Turtles. Literature Cited Linnaeus Fund Research Report BUHi.MANN,K.A., AND VAUGHAN,M.R. 1991. Ecology of the turtle 12 3 Pseudemys concinna in the New River, West Virginia. J. Herpetol. PETER V. LINDEMAN • • 25:72-78. CAGLE,F.R. 1939. A system for marking turtles for future identifica 1Center for Reservoir Research, Murray State University, tion. Copeia 1939:170-173 . Murray, Kentucky 42071 USA; 2Department of Biology, CAHN,A.R. 1937. Turtles oflllinois. Illinois Biol. Monogr . 16: 1-218. University of Louisville, Louisville, Kentucky 40292 USA; CONGDON,J.D., DUNHAM,A.E., AND VAN LoBEN Sas, R.C. 1993. 3Present Address: Division of Biological Sciences and Delayed sexual maturity and demographics of Blanding' s turtles Related Technologies, Madisonville Community College, (Emydoidea blandingii): implications for conservation and man 2000 College Drive, Madisonville, Kentucky 42431 USA agement oflong-lived organisms. -
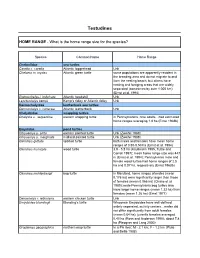
Home Range Testudines
Testudines HOME RANGE - What is the home range size for the species? Species Common Name Home Range Cheloniidae sea turtles Caretta c. caretta Atlantic loggerhead Unk Chelonia m. mydas Atlantic green turtle some populations are apparently resident in the breeding area and do not migrate to and from the nesting beach, but others have nesting and foraging areas that are widely separated (sometimes by over 1,000 km) (Ernst et al. 1994) Eretmochelys i. imbricata Atlantic hawksbill Unk Lepidochelys kempii Kemp’s ridley or Atlantic ridley Unk Dermochelyidae leatherback sea turtles Dermochelys c. coriacea Atlantic leatherback Unk Chelydridae snapping turtles Chelydra s. serpentina eastern snapping turtle in Pennsylvanina, nine adults…had estimated home ranges averaging 1.8 ha (Ernst 1968b) Emydidae pond turtles Chrysemys p. picta eastern painted turtle Unk (Zweifel 1989) Chrysemys p. marginata midland painted turtle Unk (Zweifel 1989) Clemmys guttata spotted turtle both males and females have mean home ranges of 0.50-0.53 ha (Ernst et al. 1994) Clemmys insculpta wood turtle 3.9 - 5.8 ha (Kaufmann 1995, Tuttle and Carroll 1997); mean home range size was 447 m (Ernst et al. 1994); Pennsylvania male and female wood turtles had home ranges of 2.5 ha and 0.07 ha, respectively (Ernst 1968b) Clemmys muhlenbergii bog turtle in Maryland, home ranges of males (mean 0.176 ha) were significantly larger than those of females (mean 0.066 ha) (Chase et al. 1989); male Pennsylvania bog turtles also have larger home ranges (mean 1.33 ha) than females (mean 1.26 ha) (Ernst 1977) Deirochelys r. -

Water Turtles
WATER TURTLES Range Bog Turtle Bog Turtle • North America’s smallest turtle (3” to 4.5”); NC’s rarest • Have noticeable bright orange, yellow or red blotch on each side of face • Live in isolated spring-fed fen, sphagnum bogs, marshy meadows and wet pastures • Eat beetles, insect larvae, snails, seeds and millipedes • Females mature from 5-8 years and mate from May to Yellow-bellied Slider June Range • Deposit 2-6 eggs from June to July which hatch after 42 -56 days of incubation • Placed on NC’s threatened list in 1989 ENDANGERED 1 Yellow-bellied Slider • Yellow patch on side of head – on females and juveniles • 5 to 8 inches • Underside of shell is yellow • Males smaller than females with longer, thicker tail and long fingernails • Juveniles eat more carnivorous diet Redbelly Turtle • Adults are omnivores – feeding = underwater • Males mature between 3-5 yrs • 5-7 year old females lay 4 to 23 eggs from May to July • Eggs incubate 2-2.5 months and hatch between July- September • Help control invertebrate and vegetation populations • Live in fresh water Range Red Belly Turtle • 10-15” in length • Like deep water • Eat both plants and animals-insect larvae, crayfish, worms and tadpoles • Active from May thru October Snapping Turtle • Females lay 10-12 eggs in June-July which hatch Range sometime in late summer. • Young sometimes winter over in the nest until spring • Like to bask in the sun 2 Snapping Turtle • 8-14 inches weighing from 10 to 50 lbs • Large head, small plastron, long tail & strong limbs • Males larger than females Range -

Chelonian Advisory Group Regional Collection Plan 4Th Edition December 2015
Association of Zoos and Aquariums (AZA) Chelonian Advisory Group Regional Collection Plan 4th Edition December 2015 Editor Chelonian TAG Steering Committee 1 TABLE OF CONTENTS Introduction Mission ...................................................................................................................................... 3 Steering Committee Structure ........................................................................................................... 3 Officers, Steering Committee Members, and Advisors ..................................................................... 4 Taxonomic Scope ............................................................................................................................. 6 Space Analysis Space .......................................................................................................................................... 6 Survey ........................................................................................................................................ 6 Current and Potential Holding Table Results ............................................................................. 8 Species Selection Process Process ..................................................................................................................................... 11 Decision Tree ........................................................................................................................... 13 Decision Tree Results .............................................................................................................