Pertussis Surveillance in Sweden, Twenty-Year Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thorne Moors :A Palaeoecological Study of A
T...o"..e MO<J "S " "",Ae Oe COlOOIC'" S T<.OY OF A e"ONZE AGE slTE - .. "c euc~ , A"O a • n ,• THORNE MOORS :A PALAEOECOLOGICAL STUDY OF A BRONZE AGE SITE A contribution to the history of the British Insect fauna P.c. Buckland, Department of Geography, University of Birmingham. © Authors Copyright ISBN ~o. 0 7044 0359 5 List of Contents Page Introduction 3 Previous research 6 The archaeological evidence 10 The geological sequence 19 The samples 22 Table 1 : Insect remains from Thorne Moors 25 Environmental interpretation 41 Table 2 : Thorne Moors : Trackway site - pollen and spores from sediments beneath peat and from basal peat sample 42 Table 3 Tho~ne Moors Plants indicated by the insect record 51 Table 4 Thorne Moors pollen from upper four samples in Sphagnum peat (to current cutting surface) 64 Discussion : the flooding mechanism 65 The insect fauna : notes on particular species 73 Discussion : man, climate and the British insect fauna 134 Acknowledgements 156 Bibliography 157 List of Figures Frontispiece Pelta grossum from pupal chamber in small birch, Thorne Moors (1972). Age of specimen c. 2,500 B.P. 1. The Humberhead Levels, showing Thorne and Hatfield Moors and the principal rivers. 2 2. Thorne Moors the surface before peat extraction (1975). 5 3. Thorne Moors the same locality after peat cutting (1975). 5 4. Thorne Moors location of sites examined. 9 5. Thorne Moors plan of trackway (1972). 12 6. Thorne Moors trackway timbers exposed in new dyke section (1972) • 15 7. Thorne Moors the trackway and peat succession (1977). -

Berichte/Reports 24
Alfred Toepfer Akademie für Naturschutz Berichte/Reports 24. Jahrgang, Sonderheft 1, 2012 Vol. 24, Special Issue 1, 2012 Taraxacum officinale International Youth Federation for Environmental Studies and Conservation (IYF) An account of the involvement of young people in conservation from 1950 to 2010 NNA Ber. 24. Jg. Sonderh.1 130 S. Schneverdingen 2012 ISSN: 0935 - 1450 International Youth Federation for Environmental Studies and Conservation – An account of the involvement of young people in conservation from 1950 to 2010 Zitiervorschlag: Withrington, David (Hrsg. 2012): International Youth Federation for Environmental Studies and Conservation – an account of the involvement of young people in conservation from 1950 to 2010 – NNA-Berichte 24. Jg., Sonderh. 1, Schneverdingen,130 S. Editor and Distributor: Alfred Toepfer Akademie für Naturschutz (NNA) Hof Möhr, D-29640 Schneverdingen, Telefon (05199) 989-0, Telefax (05199) 989-46 E-Mail: [email protected] Internet: www.nna.de Responsibility for the content of each contribution is that of the respective author. Chief Editor: David Withrington, Peterborough, UK Front cover: Taraxacum is the scientific name for a widespread family of plants, whose seed is the emblem of the International Youth Federation for Environmental Studies and Conservation. It symbolises the message of conservation being carried on the wind across the world. © IYF ISSN 0935-1450 Printed on recycled paper (100% waste paper) photo: David Withrington Planning meeting for the book in September 2009. Clockwise round the table: Jan Čeřovský, Fred van der Vegte, Hein van Bohemen, Henry Makowski, Pieter Ketner, Wim van Muiswinkel, Anne Bogaerts, Gerhard Walter, Lutz Katschner, Franco Pedrotti. Acknowledgements In September 2009, a meeting of people involved in the foundation in 1956 and subsequent development of the International Youth Federation for Environmental Studies and Conservation (IYF) took place at Reinsehlen, in the Lüneburger Heide, Germany. -

Suspects & Offenders with Learning Disabilities
People with learning disabilities in the CJS Glynis Murphy Tizard Centre, Kent University [email protected] Outline Prevalence of ‘offending’ in pwld Vulnerabilities in the CJS with regard to understanding rights, being interviewed, making decisions, going to court Recent policy – Bradley et seq Assessment, treatment and support Conclusions Eugenics era (1900 on) Massive anxiety here and in USA that ‘unfit’ people were in some way ‘polluting the stock’ In UK led to institutions; in USA to involuntary sterilisation laws Publicity around e.g. Kallikak family (Goddard, 1912) Terman (1916): ‘No investigator denies the fearful role played by mental deficiency in …crime, vice & delinquency… not all criminals are feeble-minded but all feeble-minded are at least potential criminals’ Early studies Woodward 1955: Reviewed over 300 studies of crime and learning disabilities (1910 - 1950 in USA Before 1920: On average 51% of convicted criminals reported to have learning disabilities; by 1928: this was 20%; by 1950 this was 4% - why? Walker & McCabe: 90% sample of all hospital orders made by courts under MHA 1959 in 1 year (‘63-’64) in England Of 969 men: one third were detained under ‘subnormality’ & those with LD (1/3) had committed 2/3s of the sex offences and one half of the arson offences – so we can conclude??? Cohort studies of prevalence Farrington & West studies: 411 boys born in 1953. ‘Working class’, S. London. Followed up for 32 years. Over a third convicted by 32 yrs. Convictions related to cognitive ability, school achievement, large families, poor parenting, etc Hodgins 1992 & 1996: Follow-up of 2 cohorts (15,117 born 1953 in Sweden & >324,000 in Denmark) showed risk ratios for convictions : 3 to 7 times higher for men & 4 to 6 times higher for women with LD. -

Origins and Development Directions of Diamond Abrasive Tools for Processing of Stone Materials
Zeszyty Naukowe Politechniki Częstochowskiej nr 24 (2018), 291–297 DOI: 10.17512/znb.2018.1.46 Origins and development directions of diamond abrasive tools for processing of stone materials Paweł Rajczyk1 ABSTRACT: The technical level of the stone processing tools corresponds to the level of advanced technology of process- ing stone pieces of a specific degree of hardness. The analysis of historical building objects, e.g. in Ancient Egypt, informs and allows to put forward a hypothesis about the tools used at that time and the advance- ment of machining technology from the period from 3000 BC, where the small natural grains of the dia- mond, called amadeus, were used as an abrasive. The historical periods describing the diamond, as a raw material for the production of machining tools, proved that the degree of development of human civilization and the possibility of technical development of many branches of economic life in the whole world is deter- mined by the determinant of machining tools. The study presents the genesis of the development of machin- ing tools with placing and verifying the hypothesis about the production of diamond segments during the construction of the pyramids in Giza, with the 19th and 20th century development of diamond tools. The hypothesis put forward results from the conducted research studies analyzing the behavior of diamond grain in a metallic–tin bond. KEYWORDS: diamond powder; stone materials; stone processing; tool development 1. Introduction The cutting of basalt and granite in Ancient Egypt, as evidenced by the assessment of traces left on the surface of granite on the walls of sarcophagi, drill holes in granite rocks in Giza, was relatively simple in those days, a relatively simple task, which is confirmed by the traces left after the incision in granite rock. -

SE Analytical Factsheet for Sweden
version: September 2019 SE Analytical factsheet for Sweden: Nine objectives for a future Common Agricultural Policy This factsheet provides an overview of the agricultural sector and rural development in Sweden. The factsheet presents facts and figures for each of the 9 specific objectives of the Common Agricultural Policy after 2020, as proposed by the Commission on 1 June 2018 (COM(2018)392 final). The information reflects all common context indicators and impact indicators in relation to agriculture and rural development for which data is available to date. This factsheet is based on available information received from Member States by the Commission up to August 2019. It is made available without prejudice to any finding in respect of Member State compliance with the regulatory framework and does not prejudge on Member States' future CAP Strategic Plans. 1 Table of contents impact in Indicator Source Current CMEF indicator PMEF Support viable farm income and resilience across the Union to enhance food security Agricultural income versus general economy EUROSTAT yes Impact indicator I.01 Evolution of agricultural income EUROSTAT yes Impact indicators I.01 & I.02 Evolution of agricultural income by sector DG AGRI - FADN yes Evolution of agricultural income by farm size DG AGRI - FADN Evolution of agricultural income in ANC areas DG AGRI - FADN yes Enhance market orientation and increase competitiveness Total factor productivity EUROSTAT yes Impact indicator I.03 Gross fixed capital formation in agriculture EUROSTAT Context indicator -

Causes and Characteristics of Population Aging
Causes and Characteristics of Population Aging : Evidence from Korea among OECD Countries* Kyounghoon Park** May 2018 The contents of this paper represent the personal opinions of the author and do not necessarily reflect the official view of the Bank of Korea. Any report/citation of this paper should specify the name of the author. * This version is built upon the published paper in the Bank of Korea Monthly Bulletin (June 2017), The Effect of Population Aging and the Policy Challenge: ChapterⅡ-1 (September 2017) in Korean, and in the Bank of Korea Quarterly Bulletin (September 2017) in English. ** Economist, Macroeconomic and Fiscal Research Team, Research Department, Bank of Korea (Tel: +82-2-759-4427, Email: [email protected]) The author would like to express gratitude to Deputy Director Jaerang Lee of the Bank of Korea Economic Research Institute (BOKERI) and Hwankoo Kang, head of the Financial and Monetary Economics Team at the Bank of Korea (BOK), both of whom were of great help in writing this paper. The author would also like to thank Professor Jinill Kim of Korea University, Director Jong Ku Kang at the BOK, Senior economist Kiho Kim of the Macroeconomics Team at the BOK, Economist Sungyub Chung of the Industry and Labor Research Team of the Research Department at the BOK (who provided useful comments), Research associate Junseok Park (who assisted in classifying the materials), participants of the BOKERI’s seminars and the bilateral meeting of the BOK and the Deutsche Bundesbank, and anonymous reviewers. The author claims full responsibility for any errors or mistakes that remain in this paper. -

Loadings on Buildings
Loadings on buildings Autor(en): Mitchell, G.R. Objekttyp: Article Zeitschrift: IABSE reports of the working commissions = Rapports des commissions de travail AIPC = IVBH Berichte der Arbeitskommissionen Band (Jahr): 3 (1969) PDF erstellt am: 11.10.2021 Persistenter Link: http://doi.org/10.5169/seals-5009 Nutzungsbedingungen Die ETH-Bibliothek ist Anbieterin der digitalisierten Zeitschriften. Sie besitzt keine Urheberrechte an den Inhalten der Zeitschriften. Die Rechte liegen in der Regel bei den Herausgebern. Die auf der Plattform e-periodica veröffentlichten Dokumente stehen für nicht-kommerzielle Zwecke in Lehre und Forschung sowie für die private Nutzung frei zur Verfügung. Einzelne Dateien oder Ausdrucke aus diesem Angebot können zusammen mit diesen Nutzungsbedingungen und den korrekten Herkunftsbezeichnungen weitergegeben werden. Das Veröffentlichen von Bildern in Print- und Online-Publikationen ist nur mit vorheriger Genehmigung der Rechteinhaber erlaubt. Die systematische Speicherung von Teilen des elektronischen Angebots auf anderen Servern bedarf ebenfalls des schriftlichen Einverständnisses der Rechteinhaber. Haftungsausschluss Alle Angaben erfolgen ohne Gewähr für Vollständigkeit oder Richtigkeit. Es wird keine Haftung übernommen für Schäden durch die Verwendung von Informationen aus diesem Online-Angebot oder durch das Fehlen von Informationen. Dies gilt auch für Inhalte Dritter, die über dieses Angebot zugänglich sind. Ein Dienst der ETH-Bibliothek ETH Zürich, Rämistrasse 101, 8092 Zürich, Schweiz, www.library.ethz.ch http://www.e-periodica.ch -

Common Scab and Its Control in Seed-Potato Crops
R. E. Labruyère Institute of Phytopathological Research, Wageningen Common scab and its control in seed-potato crops /pudooI 1971 Centrefor AgriculturalPublishing and Documentation Wageningen ISBN 90 220 0369 8 The author graduated as Doctor of Agricultural Sciences, Agricultural University, Wageningen, the Netherlands, on a thesis with the same title and contents. This publication will also be issued as Mededeling 575 van het Instituut voor Plantenziekten- kundig Onderzoek (Wageningen, the Netherlands). © Centre for Agricultural Publishing and Documentation, Wageningen, 1971. No part of this book may be reproduced and/or published in any form, by print, photoprint, microfilm or any other means without written permission from the publishers. Abstract LABRUYÈRE, R. E. (1971) Common scab and its control in seed-potato crops. Agric. Res. Repts (Versl. landbouwk. Onderz.) 767, pp. (viii) -f 72, tables 30, figs 22. Eng. summary. ISBN 90 220 0369 8 Also: Doctoral thesis, Wageningen and Meded. Tnst. plziektenk. Onderz. 575. In the Netherlands, common scab of potato is usually caused by Streptomyces scabies. Non pathogenic strains of S. scabies exist and a pathogenicity test is the only way of distinguishing between pathogenic and non-pathogenic isolates. Three types of scab were distinguished on the variety Bintje and called: 'normal', 'superficial' and 'russet'. The anatomy of lesions and reaction to external conditions differed in normal and superficial from in russet scab. Different ways of control have been tested: by chemicals, antagonists, crop rotation and irrigation. Control by irrigation proved most effective, if the soil was kept near field capacity for four weeks after tuber formation. Irrigation altered the microbial population of the soil. -

HOC History Complete
HOC history: Part One – Foundation Let’s start at the very beginning. The problem is knowing exactly where to start. The events of April 1st 1968, which will be commemorated later this year, are not the start of this story. The small group of people who founded Harlequins Orienteering Club in a hall in Kinver on that day did not mystically appear from some sort of void; their story began earlier and is important to this account. Perhaps we need to go back a further two years to the creation of the Halesowen and District Club, following as it did in quick succession to the West Midlands’ first club, Octavian Droobers. Or do we perhaps return to 1964 or even 1962, to the respective foundations of the country’s first club (South Ribble) and the country’s first association (the Scottish). In fact, I want to start in the pages of the Observer newspaper over fifty years ago. The reason for this is that the story of Harlequins is not really about a sequence of dates and obscure facts. It is about people, a chain of key individuals who made things happen and who effectively passed the baton from one to another. One of the first people in our chain wrote these words in his Sunday column in 1957. “I have just taken part, for the first time, in one of the best sports in the world. It is hard to know what to call it. The Norwegians call it 'orientation'.....”. This was probably the first time that the sport had been given such public exposure in Britain. -

A Global Perspective on Pediatric Infections
Johanna Rubin A GLOBAL PERSPECTIVE ON Paediatrician PhD Karolinska/Södersjukhuset CHILDHOOD INFECTIONS Stockholm GLOBAL HEALTH- PUBLIC HEALTH ON A GLOBAL LEVEL? Inequalities in health; - between regions - between sexes - between agegroups CHILD MORTALITY “Despite progress over the past two decades, in 2018 alone, an estimated 6.2 million children and young adolescents under age 15 died, mostly from preventable causes. Newborns account for 2.5 million of these deaths, children aged 1−11 months for 1.5 million, children aged 1−4 years for 1.3 million, and just under 1 million deaths for children and young adolescents aged 5- 14 years.” (WHO website) Group I –other communicable, maternal, perinatal and nutritional conditions THE PEDIATRIC PERSPECTIVE PEDIATRIC INFECTIONS Pediatric perspective in medicine Panorama of pediatric infections of public health importance Immunizations Access to care Regional differences and focus areas What to remember DEFINITION OF INDICATORS IN PEDIATRIC GLOBAL HEALTH Under-five mortality rate: Probability of dying between birth and exactly 5 years of age, expressed per 1,000 live births. Infant mortality rate: Probability of dying between birth and exactly 1 year of age, expressed per 1,000 live births. Neonatal mortality rate: Probability of dying during the first 28 days of life, expressed per 1,000 live births. Probability of dying among children aged 5–14: Probability of dying at age 5–14 years expressed per 1,000 children aged 5. PEDIATRIC PERSPECTIVE IN MEDICINE ”Difficult” to treat ”Difficult” to diagnose -
Tractor Operator Roll Over Protection
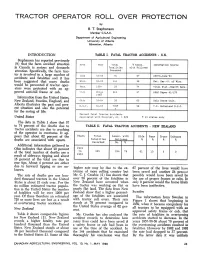
TRACTOR OPERATOR ROLL OVER PROTECTION by B. T. Stephonson Member C.S.A.E. Department of Agricultural Engineering University of Alberta Edmonton, Alberta INTRODUCTION TABLE I. FATAL TRACTOR ACCIDENTS - U.S. Stephanson has reported previously (9) that the farm accident situation Area Year Total % Assoc, Information Source in Canada is serious and demands Fatalities with Rollover attention. Specifically, the farm trac Recorded tor is involved in a large number of Iowa 53-63 54 67 AE970-June*63 accidents and fatalities and it has been suggested that many deaths Wise. 63-65 141 58 Ext. Ser.-U. of Wise. would be prevented if tractor oper ators were protected with an ap Kans . 1964 23 74 Vital Stat.-Health Kans. proved anti-roll frame or cab. Oftio Prior 212 57 ASAE Paper 61-129 196? Information from the United States, New Zealand, Sweden, England, and Ohio 59-64 39 63 Ohio State Univ. Alberta illustrates the past and pres N.S.C. 60-65 735* 58 T.D. McFarland N.S.C. ent situation and also the potential 4 for the saving of life. Percent Fatal Tractor Accidents United States Associated with Rollover, Av. = 62% * 13 states only- The data in Table 1 show that 57 to 74 percent of the deaths due to TABLE II. FATAL TRACTOR ACCIDENTS - NEW ZEALAND tractor accidents are due to crushing of the operator in overturns. It ap pears that about 62 percent of the Years Total Assoc, with Side Rear Front Unknown deaths are associated with upsets. Fatalities Rollover % % % % Recorded No. % Additional information gathered in Ohio indicates that about 40 percent 1949 of the total number of deaths are a to 335 254 76 61 10 5 0 result of sideways tipping and about 1963 15 percent of the total are due to rear tips. -

The New Radical-Right
MASARYK UNIVERSITY Faculty of Social Studies Department of Political Science The New Radical-Right: Analysis in Selected European States Diploma Thesis Bc. Kateřina Lišaníková Supervisor: Mgr. et Mgr. Petra Vejvodová, Ph.D. UČO: 397916 Security and Strategic Studies Master’s Full-Time Study Matriculation Year 2014 In Brno, 18. 12. 2016 Declaration of authorship I declare that I wrote the Diploma Thesis The New Radical-Right: Analysis in Selected European States by myself only and that I solely used the literature in the reference list. In Brno, 18.12. 2016 .................................................... Bc. Kateřina Lišaníková 1 Special thanks to: Ms. Petra Vejvodová for supervision of this Diploma Thesis and her valuable notes and thoughts through the whole process of writing. Also, special thanks to Mr. Rufus Latham from the SND Swedish National Data Service for providing me with requested dataset and to Mr. Alexander Gilder for language cooperation. 2 Content Introduction ................................................................................................................................ 7 1. Methodology ....................................................................................................................... 9 1.1. Operationalisation ...................................................................................................... 12 1.2. Conceptualisation ...................................................................................................... 15 2. Short Overview of the Parties’ History