Does Phenology Explain Plant-Pollinator Interactions at Different Latitudes? an Assessment of Its
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
APP203875 Submissions Compilation.Pdf
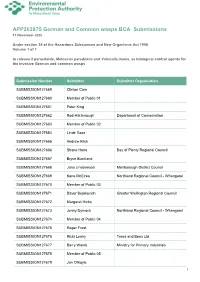
APP203875 German and Common wasps BCA Submissions 11 November 2020 Under section 34 of the Hazardous Substances and New Organisms Act 1996 Volume 1 of 1 to release 2 parasitoids, Metoecus paradoxus and Volucella inanis, as biological control agents for the invasive German and common wasps Submission Number Submitter Submitter Organisation SUBMISSION127659 Clinton Care SUBMISSION127660 Member of Public 01 SUBMISSION127661 Peter King SUBMISSION127662 Rod Hitchmough Department of Conservation SUBMISSION127663 Member of Public 02 SUBMISSION127664 Linde Rose SUBMISSION127665 Andrew Blick SUBMISSION127666 Shane Hona Bay of Plenty Regional Council SUBMISSION127667 Bryce Buckland SUBMISSION127668 Jono Underwood Marlborough District Council SUBMISSION127669 Kane McElrea Northland Regional Council - Whangarei SUBMISSION127670 Member of Public 03 SUBMISSION127671 Davor Bejakovich Greater Wellington Regional Council SUBMISSION127672 Margaret Hicks SUBMISSION127673 Jenny Dymock Northland Regional Council - Whangarei SUBMISSION127674 Member of Public 04 SUBMISSION127675 Roger Frost SUBMISSION127676 Ricki Leahy Trees and Bees Ltd SUBMISSION127677 Barry Wards Ministry for Primary Industries SUBMISSION127678 Member of Public 05 SUBMISSION127679 Jan O'Boyle 1 Submission Number Submitter Submitter Organisation Apiculture New Zealand Science and SUBMISSION127680 Sue Carter Research Focus Group SUBMISSION127681 Andrea Dorn SUBMISSION127682 Benita Wakefield Te Rūnanga o Ngāi Tahu SUBMISSION127683 Emma Edney-Browne Auckland Council SUBMISSION127684 David Hunter -

Identification of the Species of the Cheilosia Variabilis Group
Contributions to Zoology, 78 (3) 129-140 (2009) Identification of the species of the Cheilosia variabilis group (Diptera, Syrphidae) from the Balkan Peninsula using wing geometric morphometrics, with the revision of status of C. melanopa redi Vujić, 1996 Lj. Francuski1, A. Vujić1, A. Kovačević1, J. Ludosˇki1 ,V. Milankov1, 2 1 Faculty of Sciences, Department of Biology and Ecology, University of Novi Sad, Trg Dositeja Obradovića 2, 21000 Novi Sad, Serbia 2 E-mail: [email protected] Key words: Cheilosia variabilis group, geometric morphometrics, intraspecific divergent units, species delimita- tion, wing shape, wing size Abstract Recognition of phenotypic units .......................................... 134 Phenotypic relationships ....................................................... 136 The present study investigates phenotypic differentiation pat- Discussion ...................................................................................... 137 terns among four species of the Cheilosia variabilis group (Dip- Species delimitation ............................................................... 137 tera, Syrphidae) using a landmark-based geometric morphomet- Intraspecies phenotypic diversity ........................................ 138 ric approach. Herein, wing geometric morphometrics established Phenetic relationships ............................................................ 138 species boundaries that confirm C. melanopa and C. redi stat. Acknowledgements ..................................................................... -

Linear and Non-Linear Effects of Goldenrod Invasions on Native Pollinator and Plant Populations
Biol Invasions (2019) 21:947–960 https://doi.org/10.1007/s10530-018-1874-1 (0123456789().,-volV)(0123456789().,-volV) ORIGINAL PAPER Linear and non-linear effects of goldenrod invasions on native pollinator and plant populations Dawid Moron´ . Piotr Sko´rka . Magdalena Lenda . Joanna Kajzer-Bonk . Łukasz Mielczarek . Elzbieta_ Rozej-Pabijan_ . Marta Wantuch Received: 28 August 2017 / Accepted: 7 November 2018 / Published online: 19 November 2018 Ó The Author(s) 2018 Abstract The increased introduction of non-native and native plants. The species richness of native plants species to habitats is a characteristic of globalisation. decreased linearly with goldenrod cover, whereas the The impact of invading species on communities may abundance and species richness of bees and butterflies be either linearly or non-linearly related to the decreased non-linearly with increasing goldenrod invaders’ abundance in a habitat. However, non-linear cover. However, no statistically significant changes relationships with a threshold point at which the across goldenrod cover were noted for the abundance community can no longer tolerate the invasive species and species richness of hover flies. Because of the non- without loss of ecosystem functions remains poorly linear response, goldenrod had no visible impact on studied. We selected 31 wet meadow sites that bees and butterflies until it reached cover in a habitat encompassed the entire coverage spectrum of invasive of about 50% and 30–40%, respectively. Moreover, goldenrods, and surveyed the abundance and diversity changes driven by goldenrod in the plant and of pollinating insects (bees, butterflies and hover flies) D. Moron´ (&) Ł. Mielczarek Institute of Systematics and Evolution of Animals, Polish Department of Forests and Nature, Krako´w Municipal Academy of Sciences, Sławkowska 17, 31-016 Krako´w, Greenspace Authority, Reymonta 20, 30-059 Krako´w, Poland Poland e-mail: [email protected] e-mail: [email protected] P. -

White Admiral Newsletter
W h i t e A d m i r a l Newsletter 88 Summer 2014 Suffolk Naturalists’ Society C o n te n t s E d i t or i a l Ben Heather 1 Another new fungus for Suffolk Neil Mahler 2 The battle continues…the fight Matt Holden 4 against invasive alien plants in the Stour Valley! Nesting Materials Richard Stewart 8 How you can help monitor Suffolk’s Su e H o ot on 8 b a t s ? Records please! Rosemary Leaf Beetle Ben Heather 10 Stratiomys longicornis – a fly a long Peter Vincent 11 way from home! Periglacial Landforms in Breckland Caroline Markham 13 Are some roadside plants on the Dr. Anne Kell and 15 verge of extinction? Dennis Kell Where has all the road kill gone? Tom Langton 21 Back on the Hopper Trail in 2013 Colin Lucas & 22 Tricia Taylor Volucella zonaria – an impressive Peter Vincent 24 b e a s t Suffolk Show Wildlife H a w k H on e y 27 Suffolk’s Nature Strategy Nick Collinson 29 Species ‘Re - introductions’ Nick Miller 32 ISSN 0959-8537 Published by the Suffolk Naturalists’ Society c/o Ipswich Museum, High Street, Ipswich, Suffolk IP1 3QH Registered Charity No. 206084 © Suffolk Naturalists’ Society Front cover: Alder spittle bug - Aphrophora alni by Ben Heather Newsletter 88 - Summer 2014 Thank you to all those who have contributed to this full issue of the White Admiral newsletter. This issue covers a wide range of topics from roadside verges to an observation on the lack of roadkill on our roads. -

Read the Decision
Decision Date 5 February 2021 Application number APP203875 Application type To import for release and/or release from containment any new organism under section 34 of the Hazardous Substances and New Organisms Act 1996 Applicant Tasman District Council Date of hearing/consideration 17 December 2020 Date Application received 14 September 2020 Considered by A decision-making committee of the Environmental Protection Authority (the Committee)1 Dr Nick Roskruge (Chair) Dr John Taylor Mr Peter Cressey Purpose of the application To import and release two parasitoids, Metoecus paradoxus and Volucella inanis as biological control agents for the invasive German and common wasps (Vespula germanica and V. vulgaris). New organism approved Metoecus paradoxus Linnaeus 1761 Volucella inanis Linnaeus 1758 1 The Committee referred to in this decision is the subcommittee that has made the decision on this application under delegated authority in accordance with section 18A of the Act. Decision APP203875 Summary of decision 1. Application APP203875 to import and release two parasitoids, Metoecus paradoxus and Volucella inanis, as biological control agents (BCAs) for the invasive German and common wasps (Vespula germanica and V. vulgaris), in New Zealand, was lodged under section 34 of the Hazardous Substances and New Organisms Act 1996 (the Act). 2. The application was considered in accordance with the relevant provisions of the Act and of the HSNO (Methodology) Order 1998 (the Methodology). 3. The Committee has approved the application in accordance with section 38(1)(a) of the Act. Application and consideration process 4. The application was formally received on 14 September 2020. 5. The applicant, Tasman District Council, applied to the Environmental Protection Authority (EPA) to import and release two parasitoids, Metoecus paradoxus and Volucella inanis, as BCAs for the invasive German and common wasps (Vespula germanica and V. -

Appendix 1 Appendix 2
Oikos OIK-07259 de Manincor, N., Hautekeete, N., Piquot, Y., Schatz, B., Vanappelghem, C. and Massol, F. 2020. Does phenology explain plant–pollinator interactions at different latitudes? An assessment of its explanatory power in plant–hoverfly networks in French calcareous grasslands. – Oikos doi: 10.1111/oik.07259 Appendix 1 The following Supplementary material is available for this article: Figure A1. Sites location in France. Figure A2. Block clustering provided by LBM in the site of Falaises (FAL, Normandie), overlaid on a heatmap of species phenology overlap. Figure A3. Block clustering provided by LBM in the site of Bois de Fontaret (BF, Occitanie), overlaid on a heatmap of species phenology overlap. Figure A4. Block clustering provided by LBM in the site of Larris (LAR, Hauts-de-France), overlaid on a heatmap of species phenology overlap. Figure A5. Block clustering provided by LBM in the site of Riez (R, Hauts-de-France), overlaid on a heatmap of species phenology overlap. Table A1. Table of transformed plant abundances. Table A2. Table of hoverfly and plant species names and abbreviations used in the LBM Figures. Table A3. Table of model accuracy. Appendix 2 Appendix 2.1. Script modularity and latent block model analysis (LBM). Appendix 2.2. Model code. Appendix 2.3. Model script for the 16 models. 1 Appendix 1 Figure A1. Site location in France: in blue the French départements Pas-de-Calais and Somme (Hauts-de-France region), in green the départements Eure and Seine Maritime (Normandie region), in orange the départment Gard (Occitanie region). The six sites correspond to the red dots (with the sites of Fourches and Bois de Fontaret represented by the same dot due to their closeness). -

HOVERFLY NEWSLETTER Dipterists
HOVERFLY NUMBER 41 NEWSLETTER SPRING 2006 Dipterists Forum ISSN 1358-5029 As a new season begins, no doubt we are all hoping for a more productive recording year than we have had in the last three or so. Despite the frustration of recent seasons it is clear that national and international study of hoverflies is in good health, as witnessed by the success of the Leiden symposium and the Recording Scheme’s report (though the conundrum of the decline in UK records of difficult species is mystifying). New readers may wonder why the list of literature references from page 15 onwards covers publications for the year 2000 only. The reason for this is that for several issues nobody was available to compile these lists. Roger Morris kindly agreed to take on this task and to catch up for the missing years. Each newsletter for the present will include a list covering one complete year of the backlog, and since there are two newsletters per year the backlog will gradually be eliminated. Once again I thank all contributors and I welcome articles for future newsletters; these may be sent as email attachments, typed hard copy, manuscript or even dictated by phone, if you wish. Please do not forget the “Interesting Recent Records” feature, which is rather sparse in this issue. Copy for Hoverfly Newsletter No. 42 (which is expected to be issued with the Autumn 2006 Dipterists Forum Bulletin) should be sent to me: David Iliff, Green Willows, Station Road, Woodmancote, Cheltenham, Glos, GL52 9HN, (telephone 01242 674398), email: [email protected], to reach me by 20 June 2006. -

Hoverflies Family: Syrphidae
Birmingham & Black Country SPECIES ATLAS SERIES Hoverflies Family: Syrphidae Andy Slater Produced by EcoRecord Introduction Hoverflies are members of the Syrphidae family in the very large insect order Diptera ('true flies'). There are around 283 species of hoverfly found in the British Isles, and 176 of these have been recorded in Birmingham and the Black Country. This atlas contains tetrad maps of all of the species recorded in our area based on records held on the EcoRecord database. The records cover the period up to the end of 2019. Myathropa florea Cover image: Chrysotoxum festivum All illustrations and photos by Andy Slater All maps contain Contains Ordnance Survey data © Crown Copyright and database right 2020 Hoverflies Hoverflies are amongst the most colourful and charismatic insects that you might spot in your garden. They truly can be considered the gardener’s fiend as not only are they important pollinators but the larva of many species also help to control aphids! Great places to spot hoverflies are in flowery meadows on flowers such as knapweed, buttercup, hogweed or yarrow or in gardens on plants such as Canadian goldenrod, hebe or buddleia. Quite a few species are instantly recognisable while the appearance of some other species might make you doubt that it is even a hoverfly… Mimicry Many hoverfly species are excellent mimics of bees and wasps, imitating not only their colouring, but also often their shape and behaviour. Sometimes they do this to fool the bees and wasps so they can enter their nests to lay their eggs. Most species however are probably trying to fool potential predators into thinking that they are a hazardous species with a sting or foul taste, even though they are in fact harmless and perfectly edible. -

Hoverfly (Diptera: Syrphidae) Richness and Abundance Vary with Forest Stand Heterogeneity: Preliminary Evidence from a Montane Beech Fir Forest
Eur. J. Entomol. 112(4): 755–769, 2015 doi: 10.14411/eje.2015.083 ISSN 1210-5759 (print), 1802-8829 (online) Hoverfly (Diptera: Syrphidae) richness and abundance vary with forest stand heterogeneity: Preliminary evidence from a montane beech fir forest LAURENT LARRIEU 1, 2, ALAIN CABANETTES 1 and JEAN-PIERRE SARTHOU 3, 4 1 INRA, UMR1201 DYNAFOR, Chemin de Borde Rouge, Auzeville Tolosane, CS 52627, F-31326 Castanet Tolosan Cedex, France; e-mails: [email protected]; [email protected] ² CNPF/ IDF, Antenne de Toulouse, 7 chemin de la Lacade, F-31320 Auzeville Tolosane, France 3 INRA, UMR 1248 AGIR, Chemin de Borde Rouge, Auzeville Tolosane, CS 52627, F-31326 Castanet Tolosan Cedex, France; e-mail: [email protected] 4 University of Toulouse, INP-ENSAT, Avenue de l’Agrobiopôle, F-31326 Castanet Tolosan, France Key words. Diptera, Syrphidae, Abies alba, deadwood, Fagus silvatica, functional diversity, tree-microhabitats, stand heterogeneity Abstract. Hoverflies (Diptera: Syrphidae) provide crucial ecological services and are increasingly used as bioindicators in environ- mental assessment studies. Information is available for a wide range of life history traits at the species level for most Syrphidae but little is recorded about the environmental requirements of forest hoverflies at the stand scale. The aim of this study was to explore whether the structural heterogeneity of a stand influences species richness or abundance of hoverflies in a montane beech-fir forest. We used the catches of Malaise traps set in 2004 and 2007 in three stands in the French Pyrenees, selected to represent a wide range of structural heterogeneity in terms of their vertical structure, tree diversity, deadwood and tree-microhabitats. -

Encyclopedia of Social Insects
G Guests of Social Insects resources and homeostatic conditions. At the same time, successful adaptation to the inner envi- Thomas Parmentier ronment shields them from many predators that Terrestrial Ecology Unit (TEREC), Department of cannot penetrate this hostile space. Social insect Biology, Ghent University, Ghent, Belgium associates are generally known as their guests Laboratory of Socioecology and Socioevolution, or inquilines (Lat. inquilinus: tenant, lodger). KU Leuven, Leuven, Belgium Most such guests live permanently in the host’s Research Unit of Environmental and nest, while some also spend a part of their life Evolutionary Biology, Namur Institute of cycle outside of it. Guests are typically arthropods Complex Systems, and Institute of Life, Earth, associated with one of the four groups of eusocial and the Environment, University of Namur, insects. They are referred to as myrmecophiles Namur, Belgium or ant guests, termitophiles, melittophiles or bee guests, and sphecophiles or wasp guests. The term “myrmecophile” can also be used in a broad sense Synonyms to characterize any organism that depends on ants, including some bacteria, fungi, plants, aphids, Inquilines; Myrmecophiles; Nest parasites; and even birds. It is used here in the narrow Symbionts; Termitophiles sense of arthropods that associated closely with ant nests. Social insect nests may also be parasit- Social insect nests provide a rich microhabitat, ized by other social insects, commonly known as often lavishly endowed with long-lasting social parasites. Although some strategies (mainly resources, such as brood, retrieved or cultivated chemical deception) are similar, the guests of food, and nutrient-rich refuse. Moreover, nest social insects and social parasites greatly differ temperature and humidity are often strictly regu- in terms of their biology, host interaction, host lated. -

Diptera, Sy Ae)
Ce nt re fo r Eco logy & Hydrology N AT U RA L ENVIRO N M EN T RESEA RC H CO U N C IL Provisional atlas of British hover les (Diptera, Sy ae) _ Stuart G Ball & Roger K A Morris _ J O I N T NATURE CONSERVATION COMMITTEE NERC Co pyright 2000 Printed in 2000 by CRL Digital Limited ISBN I 870393 54 6 The Centre for Eco logy an d Hydrolo gy (CEI-0 is one of the Centres an d Surveys of the Natu ral Environme nt Research Council (NERC). Established in 1994, CEH is a multi-disciplinary , environmental research organisation w ith som e 600 staff an d w ell-equipp ed labo ratories and field facilities at n ine sites throughout the United Kingdom . Up u ntil Ap ril 2000, CEM co m prise d of fou r comp o nent NERC Institutes - the Institute of Hydrology (IH), the Institute of Freshw ater Eco logy (WE), the Institute of Terrestrial Eco logy (ITE), and the Institute of Virology an d Environmental Micro b iology (IVEM). From the beginning of Ap dl 2000, CEH has operated as a single institute, and the ind ividual Institute nam es have ceased to be used . CEH's mission is to "advance th e science of ecology, env ironme ntal microbiology and hyd rology th rough h igh q uality and inte rnat ionall) recognised research lead ing to better understanding and quantifia ttion of the p hysical, chem ical and b iolo gical p rocesses relating to land an d freshwater an d living organisms within the se environments". -

Diptera, Syrphidae) Collected by Malaise Trap
All publications: 1984 1. Šimić, S., Vujić, A. (1984): Composition of syrphid fauna (Diptera, Syrphidae) collected by Malaise trap. Zbornik Matice Srpske za prirodne nauke, 66: 145-153. 2. Šimić, S., Vujić, A. (1984): Prilog poznavanju faune sirfida (Diptera: Syrphidae) Vršačkih planina. Bilten Društva ekologa BiH, 1: 375-380. 1987 3. Šimić, S., Vujić, A. (1987): The Syrphid fauna (Diptera) of the Tisa basin in Yugoslavia. Tiscia, 1987: 121- 127. 4. Vujić, A. (1987): New species of genus Sphegina Meigen (Diptera: Syrphidae). Glasnik Prirodnjačkog Muzeja u Beogradu, 42: 79-83. 1990 5. Vujić, A. (1990): Genera Neoascia Williston 1886 and Sphegina Meigen 1882 (Diptera:Syrphidae) in Yugoslavia and description of species Sphegina sublatifrons sp nova. Glasnik Prirodnjačkog Muzeja u Beogradu, 45: 77-93. 6. Vujić, A., Milankov, V. (1990): Taxonomic status of species belonging to genus Criorrhina Meigen 1822 (Diptera:Syrphidae) and recorded in Yugoslavia. Glasnik Prirodnjačkog Muzeja u Beogradu, 45: 105-114. 7. Vujić, A., Radović, D. (1990): Species of genus Brachypalpus Macquarti 1834 (Diptera: Syrphidae) in Yugoslavia. Glasnik Prirodnjačkog Muzeja u Beogradu, 45: 95-104. 8. Šimić, S., Vujić, A. (1990): Species of the genus Eristalis Latreille (Diptera: Syrphidae) from a collection belonging to the Institute of Biology, Novi Sad. Glasnik Prirodnjačkog Muzeja u Beogradu, 45: 115-126. 1991 9. Vujić, A. (1991): Species of genus Brachyopa Meigen 1822 (Diptera: Syrphidae) in Yugoslavia. Glasnik Prirodnjačkog Muzeja u Beogradu, 46: 141-150. 1992 10. Radišić, P., Vujić, A., Šimić, S., Radenković, S. (1992): Pollen transport of Cheilosia grossa (Fallen, 1817) (Diptera: Syrphidae). Ekologija, 2(27): 41-46. 11. Božičić-Lothrop, B., Radović, D., Vapa, Lj., Banovački, Z., Vujić, A.: Morphological and isozyme variability of isolated populations of the species Aedes communis (De Geer, 1776) (Diptera: Culicidae).