2320-5407 Int. J. Adv. Res. 6(8), 941-948
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molluscs of the Northern Mariana Islands, with Special Reference to the Selectivity of Oceanic Dispersal Barriers1
Molluscs of the Northern Mariana Islands, With Special Reference to the Selectivity of Oceanic Dispersal Barriers1 GEERAT J. VERMEIJ Department of Zoology, University of Maryland, College Park, Maryland 20742 E. ALISON KAY Department of Zoology, University of Hawaii, Honolulu, Hawaii 96822 LUCIUS G. ELDREDGE University of Guam Marine Laboratory, UOG Station, Mangilao, Guam 96913 Abstract- The shelled molluscan fauna of the Northern Marianas, a chain of volcanic islands in the tropical western Pacific, consists of at least 300 species. Of these, 18 are unknown from or are very rare in the biologically better known southern Marianas. These northern-restricted species are over-represented among limpets and in the middle to high intertidal zones of the northern Marianas. At least 22 gastropods which are common in the intertidal zone and on reef flats of the southern Marianas are absent in the northern Marianas. The northern Marianas lie within the presumed source area of the planktonically derived part of the Hawaiian marine fauna. The ocean barrier between the northern Marianas and the Hawaiian chain appears to select against archaeogastropods and against intertidal species but is unselective with respect to adult size and to other aspects of gastropod shell architecture. These findings are consistent with those for other dispersal barriers. Introduction The Mariana Islands form the southern part of an island chain which extends northward through the Bonin, Volcano, and lzu Islands to central Honshu, Japan. Whereas the marine biota of the southern Marianas (Guam, Rota, Tinian, and Saipan) is becoming relatively well known, that of the northern Marianas (Fig. 1) is largely unstudied. -

Part 5. Mollusca, Gastropoda, Muricidae
Results of the Rumphius Biohistorical Expedition to Ambon (1990) Part 5. Mollusca, Gastropoda, Muricidae R. Houart Houart, R. Results of the Rumphius Biohistorical Expedition to Ambon (1990). Part 5. Mollusca, Gas• tropoda, Muricidae. Zool. Med. Leiden 70 (26), xx.xii.1996: 377-397, figs. 1-34.— ISSN 0024-0672. Roland Houart, research associate, Institut Royal des Sciences Naturelles de Belgique, Rue Vautier, 29, B-1000 Bruxelles. Key words: Rumphius expedition; Indonesia; Ambon; Mollusca; Gastropoda; Muricidae. This report deals with the species of Muricidae collected during the Rumphius Biohistorical Expedi• tion on Ambon. At 44 stations, a total of 58 species in 23 genera of the prosobranch gastropod family Muricidae were obtained. The identity and the classification of several nominal taxa is revised. Four species are new to science and are described here: Pygmaepterys cracentis spec. nov. (Muricopsinae), Pascula ambonensis spec. nov. (Ergalataxinae), Thais hadrolineae spec. nov. and Morula rumphiusi spec, nov. (Rapaninae). Some comments are included on the Muricidae described by Rumphius and on their identification. In his "Amboinsche Rariteitkamer" (1705) 21 species of muricids were included, of which 16 were illu• strated by Rumphius or Schijnvoet. The identity of those species is determined. During the Rumphius Biohistorical Expedition seven of these species have been refound. Introduction The Rumphius Biohistorical Expedition in Ambon (Moluccas, Indonesia) was held from 4 November till 14 December, 1990. The primary goal of the expedition was to collect marine invertebrates on the localities mentioned by Rumphius (1705). The general account, with a list of stations, was published by Strack (1993); it con• tains the history of the expedition and a detailed description of each station, with photographs. -

Checklist of the Mollusca of Cocos (Keeling) / Christmas Island Ecoregion
RAFFLES BULLETIN OF ZOOLOGY 2014 RAFFLES BULLETIN OF ZOOLOGY Supplement No. 30: 313–375 Date of publication: 25 December 2014 http://zoobank.org/urn:lsid:zoobank.org:pub:52341BDF-BF85-42A3-B1E9-44DADC011634 Checklist of the Mollusca of Cocos (Keeling) / Christmas Island ecoregion Siong Kiat Tan* & Martyn E. Y. Low Abstract. An annotated checklist of the Mollusca from the Australian Indian Ocean Territories (IOT) of Christmas Island (Indian Ocean) and the Cocos (Keeling) Islands is presented. The checklist combines data from all previous studies and new material collected during the recent Christmas Island Expeditions organised by the Lee Kong Chian Natural History Museum (formerly the Raffles Museum of Biodiversty Resarch), Singapore. The checklist provides an overview of the diversity of the malacofauna occurring in the Cocos (Keeling) / Christmas Island ecoregion. A total of 1,178 species representing 165 families are documented, with 760 (in 130 families) and 757 (in 126 families) species recorded from Christmas Island and the Cocos (Keeling) Islands, respectively. Forty-five species (or 3.8%) of these species are endemic to the Australian IOT. Fifty-seven molluscan records for this ecoregion are herein published for the first time. We also briefly discuss historical patterns of discovery and endemism in the malacofauna of the Australian IOT. Key words. Mollusca, Polyplacophora, Bivalvia, Gastropoda, Christmas Island, Cocos (Keeling) Islands, Indian Ocean INTRODUCTION The Cocos (Keeling) Islands, which comprise North Keeling Island (a single island atoll) and the South Keeling Christmas Island (Indian Ocean) (hereafter CI) and the Cocos Islands (an atoll consisting of more than 20 islets including (Keeling) Islands (hereafter CK) comprise the Australian Horsburgh Island, West Island, Direction Island, Home Indian Ocean Territories (IOT). -

Prosobranch Gastropods of Guam
Micronesica 35-36:244-270. 2003 Prosobranch gastropods of Guam BARRY D. SMITH Marine Laboratory University of Guam Mangilao, Guam 96923 U.S.A. email: [email protected] Abstract—Based on records from invertebrate collections at the University of Guam, specimens cataloged at other institutions, and the published literature, there are 895 species of prosobranch gastropods from Guam. The vast majority of the species are marine, but terrestrial and aquatic prosobranchs are included. Most the species recorded to date are conspicuous, epibenthic species from shallow reef habitats, but some species have been taken from depths up to 400 m. Microgastropods less than 7 mm in size have been poorly investigated to date. Comparison of prosobranch gastropods from Guam and Enewetak reveal that some 56% of the species occurring at Enewetak are found in Guam. Introduction Molluscs have been collected in Guam since the arrival of the earliest inhabitants (Thompson, 1945). Despite the long history of European contact with the island, scant attention was given to systematic investigation of the fauna until the collections of Quoy and Gaimard (1824–1826; 1830–1834). Hidalgo (1904– 1905) was the first to produce a catalog that included molluscs from Guam, but his emphasis was mostly on the Philippine Islands fauna. This catalog was followed by a series of unpublished lists produced by shell collectors and shell club members during the last several decades. Synoptic collections of molluscs from Guam and Micronesia were started by faculty of the University of Guam in the mid-1960s. These collections are housed in the Richard E. Dickinson Memorial Mollusc Collection at the University of Guam Marine Laboratory. -

The Indole Pigments of Mollusca
Annls Soc. r. zool. Belg. - T. 119 (1989) - fase. 2 - pp. 181-197 - Bruxelles 1989 (Manuscript received on 31 J uly 1989) THE INDOLE PIGMENTS OF MOLLUSCA by ANDRÉ VERHECKEN Recent Invertebrates Section Koninklijk Belgisch Instituut voor Natuurwetenschappen Vautierstraat 29, B-1040 Brussels SUMMARY This study discusses occurrence and biosynthesis of melanins and indigoids, pigments with indole structure produced by mollusca. Indigotin and 6,6'-dibromoindigotin (DBI), constituents of<< mollusc purple •> , are treated in some detail. Their colourless precursors in the organism are presumed to be of dietary origin. The hypothesis is formulated that for mation of DBI is due to a detoxification mechanism ; it is suggested that purple-producing species have developed it into an enzymatically controlled defensive system against large predators. This first reaction step is then foliowed by spontaneously proceeding reactions invalving oxygen and, for DBI, light, leading to the formation of the coloured pigment, which seems to be of no further use to the anima!. Experiments with purple from three Mediterranean species are reported. Keywords : melanin, mollusc purple, indigotin, biosynthesis. RÉSUMÉ Cette étude discute l'occurence et la biosynthèse des mélanines et indigoides, pigments à structure indolique produits par des mollusques. Les indigoides indigotine et 6,6' -dibro meindigotine (DBI), présents dans la <<.pourpre des mollusques •>, sont traités en plus de détail. Les précurseurs incolores présents dans !'organisme sont supposés être d 'origine dié tique. L 'hypothèse est formulée que la formation du DBI est due à un méchanisme de détoxification, et que les espèces produisant la pourpre ont développé ce méchanisme en un système enzymatiquement contrölé de défense contre de grands prédateurs. -

Chicoreus Ramosus Are from Walter Paine
Page 2 Vol. 42, No. 1 In 1972, a group of shell collectors saw the need for a national organization devoted to the interests of shell collec- tors; to the beauty of shells, to their scientific aspects, and to the collecting and preservation of mollusks. This was the start of COA. Our member- AMERICAN CONCHOLOGIST, the official publication of the Conchol- ship includes novices, advanced collectors, scientists, and shell dealers ogists of America, Inc., and issued as part of membership dues, is published from around the world. In 1995, COA adopted a conservation resolution: quarterly in March, June, September, and December, printed by JOHNSON Whereas there are an estimated 100,000 species of living mollusks, many PRESS OF AMERICA, INC. (JPA), 800 N. Court St., P.O. Box 592, Pontiac, IL 61764. All correspondence should go to the Editor. ISSN 1072-2440. of great economic, ecological, and cultural importance to humans and Articles in AMERICAN CONCHOLOGIST may be reproduced with whereas habitat destruction and commercial fisheries have had serious ef- proper credit. We solicit comments, letters, and articles of interest to shell fects on mollusk populations worldwide, and whereas modern conchology collectors, subject to editing. Opinions expressed in “signed” articles are continues the tradition of amateur naturalists exploring and documenting those of the authors, and are not necessarily the opinions of Conchologists the natural world, be it resolved that the Conchologists of America endors- of America. All correspondence pertaining to articles published herein es responsible scientific collecting as a means of monitoring the status of or generated by reproduction of said articles should be directed to the Edi- mollusk species and populations and promoting informed decision making tor. -

Shellfish Resources Around Madras Atomic Power Station Kalpakkam
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Journal of Asia-Pacific Biodiversity xxx (2016) 1e6 HOSTED BY Contents lists available at ScienceDirect Journal of Asia-Pacific Biodiversity journal homepage: http://www.elsevier.com/locate/japb Original article Shellfish resources around Madras Atomic Power Station Kalpakkam, Southeast India K. Ponnusamy a, S. Munilkumar a, Subhashree Das a, Amrata Verma a, R. Venkitesan b, A.K. Pal a,* a ICAR-Central Institute of Fisheries Education, Mumbai, India b Zoological Survey of India, Southern Regional Centre, Chennai, India article info abstract Article history: A survey has been made to identify and assess the shellfish resources potential along the Kalpakkam Received 24 January 2016 coastal waters. Stratified random sampling was used and the collected shellfish were identified down to Received in revised form species level. A total of 108 species belonging to 18 species of crustaceans, 86 species of molluscs, and 4 28 March 2016 species of echinoderms were identified. A complete checklist of the entire marine faunal information Accepted 9 April 2016 around Kalpakkam coast is not available yet. Therefore, the present work was carried out and collected Available online xxx available information of marine shellfish around Madras Atomic Power Station (MAPS), Kalpakkam sites, India. In addition, the present checklist will provide baseline information to ecologists and taxonomists Keywords: fi crustaceans in future systematic work with respect to shell sh resources around MAPS. Ó echinoderms Copyright 2016, National Science Museum of Korea (NSMK) and Korea National Arboretum (KNA). Kalpakkam Production and hosting by Elsevier. -
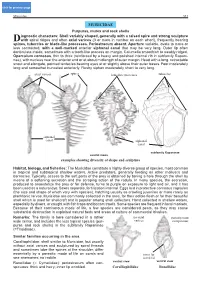
MURICIDAE Purpuras, Murex and Rock Shells CLICK FOR
click for previous page Muricidae 553 Muricidae MURICIDAE Purpuras, murex and rock shells iagnostic characters: Shell variably shaped, generally with a raised spire and strong sculpture Dwith spiral ridges and often axial varices (3 or more in number on each whorl), frequently bearing spines, tubercles or blade-like processes. Periostracum absent. Aperture variable, ovate to more or less contracted, with a well-marked anterior siphonal canal that may be very long. Outer lip often denticulate inside, sometimes with a tooth-like process on margin. Columella smoothish to weakly ridged. Operculum corneous, thin to thick (reinforced by a heavy and polished internal rib in subfamily Rapani- nae), with nucleus near the anterior end or at about midlength of outer margin. Head with a long, retractable snout and elongate, pointed tentacles bearing eyes at or slightly above their outer bases. Foot moderately long and somewhat truncated anteriorly. Fleshy siphon moderately short to very long. axial varices spines lamellate processes tubercles siphonal canal siphonal canal subfamily Rapaninae ventral views examples showing diversity of shape and sculpture Habitat, biology, and fisheries: The Muricidae constitute a highly diverse group of species, most common in tropical and subtropical shallow waters. Active predators, generally feeding on other molluscs and barnacles. Typically, access to the soft parts of the prey is obtained by boring a hole through the shell by means of a softening secretion and the scraping action of the radula. In many species, the secretion, produced to anaestetize the prey or for defense, turns to purple on exposure to light and air, and it has been used as a natural dye. -

Distribution-And-Diversity-Of-Shellfishes-Along-The-Intertidal-Rocky-Coast-Of-Veraval-Gujarat
IJSAR, 2(9), 2015; 33-39 International Journal of Sciences & Applied Research www.ijsar.in Distribution and diversity of Shellfishes along the intertidal rocky coast of Veraval, Gujarat (India) T.H. Dave3*, B.G. Chudasama1, A.S. Kotiya2, A.J. Bhatt3, D.T. Vaghela4 1Department of Harvest and Post-Harvest, College of Fisheries, JAU, Veraval, Gujarat, India, 2Agricultural Research Station, JAU, Mahuva, Gujarat, India, 3Department of Fisheries Resource management, College of Fisheries, Junagadh Agricultural University (JAU), Veraval, Gujarat, India. 4Department of Aquatic Environment, College of Fisheries, JAU, Veraval, Gujarat, India. Correspondence Address: *T.H. Dave, Department of Fisheries Resource Management, College of Fisheries, Junagadh Agricultural University (JAU), Veraval, Gujarat, India. _____________________________________________________________________________________________ Abstract The rocky intertidal coast of veraval harbours a good number of seaweeds and shellfish diversity. The vertical exposed coastal length of 60-70 meter were divided into three different zones from the highest high tide line. The zones were Zone-1 (0-20mt), Zone-2 (20-40mt), and Zone-3 (40- 60mt). The highest density of organisms found at Zone-2, followed by Zone-3 and Zone-1. The coast inhabits 33 species of shellfishes of which 28 were molluscs and 05 crustaceans. It is also observed that the most abundant and year round species observed was Patella radiate followed by Turbo intercostalis, Chiton granoradiatus, Rinoclavis sinensis and Cerithium spp. of molluscs and Balanus amphtrite among the crutaceans. The shellfishes like Hexaplex endivia, Pyrene versicolor, Anadara Spp., Murex tribulus and Chicoreus ramosus shown rare availability at almost all the level. The seawater parameters recorded were: pH – 8.2 to 8.32, Temperature – 28 to 310C, Salinity – 34.1 to 35.5 ppt, and Dissolved Oxygen – 4.8 to 5.9 mg/l. -

Muricidae (Mollusca, Neogastropoda)
DIPARTIMENTO DI BIOLOGIA E BIOTECNOLOGIE “CHARLES DARWIN” UNIVERSITÀ DI ROMA “LA SAPIENZA” CORSO DI DOTTORATO IN BIOLOGIA ANIMALE XXIII CICLO (2007 – 2010) Andrea Barco TESI DI DOTTORATO DI RICERCA I MURICIDAE (MOLLUSCA, NEOGASTROPODA): FILOGENESI MOLECOLARE, EVOLUZIONE MORFOLOGICA ED ECOLOGIA TROFICA PhD Dissertation THE MURICIDAE (MOLLUSCA, NEOGASTROPODA): MOLECULAR PHYLOGENY, MORPHOLOGICAL EVOLUTION AND TROPHIC ECOLOGY Roma, 2010 0 1 SOMMARIO RIASSUNTO 5 ABSTRACT 7 1 INTRODUZIONE 11 1.1 FILOGENESI E CLASSIFICAZIONE DEI MURICIDAE 12 1.1.1 CRONOLOGIA DELLA CLASSIFICAZIONE TRADIZIONALE 12 1.1.2 RECENTI IPOTESI FILOGENETICHE 13 1.2 LA DIVERSIFICAZIONE DEI NEOGASTEROPODI E L’ORIGINE DEI MURICIDAE 19 1.3 LA RADULA DEI MURICIDI E IL SUO ROLO NELLA PREDAZIONE 23 1.3.1 LA MORFOLOGIA RADULARE 23 1.3.2 LA STRATEGIA TROFICA 27 1.4 OBIETTIVI 30 2 MATERIALI E METODI 31 2.1 RACCOLTA E GESTIONE DEL CAMPIONE 31 2.2 ESTRAZIONE E AMPLIFICAZIONE DEL DNA 33 2.3 ESTRAZIONE E PREPARAZIONE DELLE RADULE 34 2.4 ANALISI DEI DATI 35 2.4.1 RAPPRESENTATIVITÀ FILOGENETICA DEL CAMPIONE 35 2.4.2 ANALISI DELLE SEQUENZE 36 2.4.3 ANALISI FILOGENETICHE DEL DATASET MOLECOLARE 38 2.4.4 DATAZIONE DEI CLADI CON UN OROLOGIO MOLECOLARE 39 2.4.5 ANALISI FILOGENETICHE DEL DATASET MORFOLOGICO 40 2.4.6 ANALISI COMPARATIVA DELLE STRATEGIE TROFICHE 40 3 RISULTATI 49 3.1 RAPPRESENTATIVITÀ FILOGENETICA DEL CAMPIONE 49 3.2 ANALISI DEL DATASET MOLECOLARE 50 3.2.1 ANALISI DELLE SEQUENZE 50 3.2.2 ANALISI DEI SINGOLI GENI 50 3.2.3 SCELTA DELLO SCHEMA DI PARTIZIONE E RISULTATI DELL‟ANALISI -

Environmental Assessment for Annual Catch Limit Specifications and Accountability Measures for Pacific Islands Coral Reef Ecosystem Fisheries in 2012 and 2013
U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration NATIONAL MARINE FISHERIES SERVICE Pacific Islands Regional Office 1601 Kapiolani Blvd., Suite 1110 Honolulu, Hawaii 96814-4700 (808) 944-2200 ● Fax (808) 973-2941 Environmental Assessment for Annual Catch Limit Specifications and Accountability Measures for Pacific Islands Coral Reef Ecosystem Fisheries in 2012 and 2013 Including a Regulatory Impact Review December 13, 2011 Responsible Agency: Pacific Islands Regional Office (PIRO) National Marine Fisheries Service (NMFS) National Oceanic and Atmospheric Administration (NOAA) Responsible Official: Michael D. Tosatto Regional Administrator NMFS PIRO 1601 Kapiolani Blvd., Suite 1110 Honolulu, HI 96814 (808) 944-2200 Responsible Council: Western Pacific Fishery Management Council 1164 Bishop St., Suite 1400 Honolulu, HI 96813 Contact: Kitty M. Simonds Executive Director (808)522-8220 Abstract NMFS proposes to specify an annual catch limit (ACL) and accountability measures (AM) for each coral reef ecosystem stock and stock complex of management unit species (MUS) in American Samoa, Guam, the Northern Mariana Islands, and Hawaii. The ACLs and AMs would be applicable in fishing years 2012 and 2013 which begin January 1 and end December 31, annually. The purpose of the action is to comply with provisions of the fishery ecosystem plans (FEP) for American Samoa, the Mariana Archipelago, and Hawaii which require NMFS to specify an ACL for each stock and stock complex in western Pacific coral reef ecosystem fisheries and implement AMs that prevent ACLs from being exceeded, and correct or mitigate overages of ACLs if they occur. Given the number of individual coral reef ecosystem stocks and stock complexes in each island area, individual species were aggregated into higher taxonomic groups, generally at the family level. -

10. Observations on the Northern Moluccan Excavated Animal Bone and Shell Collections 137 Stage 7 Is Where the Entire Surface of the Tooth Is a Dentine ‘Pool’
10 Observations on the Northern Moluccan excavated animal bone and shell collections Jennifer R. Hull, Philip Piper, Geoffrey Irwin, Katherine Szabó, Annette Oertle, and Peter Bellwood Due to their isolation, the Northern Moluccan islands today contain only a very impoverished and mainly marsupial indigenous Wallacean vertebrate fauna that includes the cuscuses Phalanger ornatus and Phalanger alexandriae (Flannery and Boeadi 1995), the sugar glider Petaurus breviceps (not found so far in any archaeological contexts in the Northern Moluccas), and the large placental rodent Rattus morotaiensis (Flannery 1995a). These species have been supplemented by several humanly mediated introductions of placental mammal species, such as Rattus exulans, R. tanezumi, Suncus marinus, Paradoxurus hermaphroditus, Cervis timorensis, plus pigs and dogs (Flannery et al. 1995). The 1990s excavations in the Moluccas produced several substantial vertebrate assemblages dating to the late Pleistocene and Holocene. The initial report on the assemblage from Gua Siti Nafisah on Halmahera was published in 1995 (Flannery et al. 1995), in which an extinct wallaby (Dorcopsis sp.) and a bandicoot related to the Echymipera/Rhynchomeles group found in the Holocene deposits of that cave were described as ‘quite unexpected’, and reported as occurring with the extant Moluccan cuscus species Phalanger ornatus. The opinion was expressed that the two extinct marsupial species were probably ‘indigenous elements in the fauna’. The succeeding report (Flannery et al. 1998) announced the further discovery of wallaby bones in Golo Cave and Wetef rockshelter on Gebe, and of Rattus morotaiensis on Morotai. This second report established that the wallaby was not found on Kayoa or Morotai (hence, so far only on Halmahera and Gebe), and that the bandicoot was only found in Gua Siti Nafisah on Halmahera.