Walter Sisulu University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Faculty of Health Sciences, Walter Sisulu University: Training Doctors from and for Rural South African Communities
Original Scientific Articles Medical Education Faculty of Health Sciences, Walter Sisulu University: Training Doctors from and for Rural South African Communities Jehu E. Iputo, MBChB, PhD ABSTRACT Outcomes To date, 745 doctors (72% black Africans) have graduated Introduction The South African health system has disturbing in- from the program, and 511 students (83% black Africans) are currently equalities, namely few black doctors, a wide divide between urban enrolled. After the PBL/CBE curriculum was adopted, the attrition rate and rural sectors, and also between private and public services. Most for black students dropped from 23% to <10%. The progression rate medical training programs in the country consider only applicants with rose from 67% to >80%, and the proportion of students graduating higher-grade preparation in mathematics and physical science, while within the minimum period rose from 55% to >70%. Many graduates most secondary schools in black communities have limited capacity are still completing internships or post-graduate training, but prelimi- to teach these subjects and offer them at standard grade level. The nary research shows that 36% percent of graduates practice in small Faculty of Health Sciences at Walter Sisulu University (WSU) was towns and rural settings. Further research is underway to evaluate established in 1985 to help address these inequities and to produce the impact of their training on health services in rural Eastern Cape physicians capable of providing quality health care in rural South Af- Province and elsewhere in South Africa. rican communities. Conclusions The WSU program increased access to medical edu- Intervention Access to the physician training program was broad- cation for black students who lacked opportunities to take advanced ened by admitting students who obtained at least Grade C (60%) in math and science courses prior to enrolling in medical school. -

The Power of Heritage to the People
How history Make the ARTS your BUSINESS becomes heritage Milestones in the national heritage programme The power of heritage to the people New poetry by Keorapetse Kgositsile, Interview with Sonwabile Mancotywa Barbara Schreiner and Frank Meintjies The Work of Art in a Changing Light: focus on Pitika Ntuli Exclusive book excerpt from Robert Sobukwe, in a class of his own ARTivist Magazine by Thami ka Plaatjie Issue 1 Vol. 1 2013 ISSN 2307-6577 01 heritage edition 9 772307 657003 Vusithemba Ndima He lectured at UNISA and joined DACST in 1997. He soon rose to Chief Director of Heritage. He was appointed DDG of Heritage and Archives in 2013 at DAC (Department of editorial Arts and Culture). Adv. Sonwabile Mancotywa He studied Law at the University of Transkei elcome to the Artivist. An artivist according to and was a student activist, became the Wikipedia is a portmanteau word combining youngest MEC in Arts and Culture. He was “art” and “activist”. appointed the first CEO of the National W Heritage Council. In It’s Bigger Than Hip Hop by M.K. Asante. Jr Asante writes that the artivist “merges commitment to freedom and Thami Ka Plaatjie justice with the pen, the lens, the brush, the voice, the body He is a political activist and leader, an and the imagination. The artivist knows that to make an academic, a historian and a writer. He is a observation is to have an obligation.” former history lecturer and registrar at Vista University. He was deputy chairperson of the SABC Board. He heads the Pan African In the South African context this also means that we cannot Foundation. -

Walter Sisulu University General Prospectus 2020
WALTER SISULU UNIVERSITY GENERAL PROSPECTUS 2020 General Rules and Regulations www.wsu.ac.za GENERAL PROSPECTUS 2020 This General Prospectus applies to all four campuses of Walter Sisulu University. LEGAL RULES 1. The University may in each year amend its rules. 2. The rules, including the amended rules, are indicated in the 2020 Prospectus. 3. The rules indicated in the 2020 Prospectus will apply to each student registered at Walter Sisulu University for 2020. 4. These rules will apply to each student, notwithstanding whether the student had first registered at the University prior to 2020. 5. When a student registers in 2020, the student accepts to be bound by the rules indicated in the 2020 prospectus. 6. The University may amend its rules after the General Prospectus has been printed. Should the University amend its rules during 2020, the amended rules will be communicated to students. Students will be bound by such amended rules. CAMPUSES & FACULTIES MTHATHA CAMPUS 1. Faculty of Commerce & Administration 2. Faculty of Educational Sciences 3. Faculty of Health Sciences 4. Faculty of Humanities, Social Sciences & Law 5. Faculty of Natural Sciences BUTTERWORTH CAMPUS 1. Faculty of Education 2. Faculty of Engineering & Technology 3. Faculty of Management Sciences BUFFALO CITY CAMPUS 1. Faculty of Business Sciences 2. Faculty of Science, Engineering & Technology QUEENSTOWN CAMPUS 1. Faculty of Economics & Information Technology Systems 2. Faculty of Education & School Development 1 2020 PROSPECTUS ALL CORRESPONDENCE TO BE ADDRESSED TO: -

The Higher Education Landscape Under Apartheid
CHAPTER 2 IAN BUNTING THE HIGHER EDUCATION LANDSCAPE UNDER APARTHEID This chapter lays out the South African higher education landscape as it was shaped by the apartheid policies of the National Party government prior to 1994. It describes how the disenfranchisement of the African majority culminated in the establishment of five separate legislative and geographic entities (the Republic of South Africa and four ‘independent republics’) and traces the process by which this policy led to the establishment of 36 higher education institutions controlled by eight different government departments. The chapter also describes the apartheid thinking which led to the differentiation of higher education in South Africa into two distinct types – universities and technikons – and shows how sharp racial divisions, as well as language and culture, skewed the profiles of the institutions in each category. 1. POLICIES OF THE APARTHEID GOVERNMENT 1.1. Racial divisions in South Africa At the beginning of 1994, South Africa’s higher education system was fragmented and unco-ordinated. This was primarily the result of the white apartheid government’s conception of race and the politics of race, which had shaped the higher education policy framework that it laid down during the 1980s. The apartheid government, under the influence of the ruling National Party, had, by the beginning of the 1980s, divided South Africa into five entities: · The Republic of Transkei (formed from part of the old Cape Province). · The Republic of Bophuthatswana (formed from part of the old Transvaal Province). · The Republic of Venda (also formed from part of the old Transvaal Province). · The Republic of Ciskei (formed from another part of the old Cape Province). -

“Restructuring in South African Universities”
On The Outsourced University: A survey of the rise of support service outsourcing in public sector higher education in South Africa, and its effects on workers and trade unions, 1994-2001 FINAL REPORT May 2002 Lucien van der Walt, Chris Bolsmann, Bernadette Johnson, and Lindsey Martin Funded by the Centre for Higher Education Transformation, UNISA Research undertaken by the Sociology of Work Unit, University of the Witwatersrand Address communications to: Lucien van der Walt Department of Sociology School of Social Sciences Faculty of Humanities University of the Witwatersrand Private Bag 3 Johannesburg South Africa [email protected] Tel: +27 (0) 11 717 4441 Fax: +27 (0) 11 339 8163 Outsourcing support services in public sector higher education in South Africa, 1994-2001 RESEARCH TEAM: Lucien van der Walt, Sociology of Work Unit, University of the Witwatersrand: coordination, planning, final data analysis and write-up Chris Bolsmann, Department of Sociology, RAU: planning, initial data preparation and analysis Bernadette Johnson, School of Education, University of the Witwatersrand: planning, analysis, write-up Lindsey Martin, Department of Sociology, RAU: planning, final data analysis ADDITIONAL RESEARCHERS: Literature survey: Patrick Connolly Interviews: Shaheen Buckus. Lindsey Martin, Papi Nkoli Final data preparation and analysis: Lindsey Martin, Lucien van der Walt OTHER ACKNOWLEDGEMENTS: Glenn Adler, Nico Cloete, Khayaat Fakier, Jane Kabaki, Eddie Webster This research was made possible through the financial support of the -

Mandela Memories: an African Prometheus2
Mandela Memories: An African Prometheus2 I first met Mandela in 1991 in Johannesburg, at the offices of the ANC during my visit to South Africa, while a guest of the Congress of South African writers, who had invited me to talk at various community cen- ters to share ideas and experiences in the unfolding postapartheid demo- cratic process. Mandela had just resumed the presidency of the ANC after twenty- seven years in prison. I could never have imagined that my very first engagement in the country would be with the legend of that struggle. Mandela had been part of my literary and political imagination since his days as the Black Pimpernel who, time and again, made a fool of the pursuing apartheid police. A Makerere student at the time, I had just read Orczy’s novel The Scarlet Pimpernel, set during the French Revolution, and it was easy to equate the French reign of terror with apartheid’s and Man- dela with the Percy character, the master of disguises and elusive moves. The real Mandela of the Rivonia trial, Robben Island, and worldwide celeb- rity added to the legend. He had been the subject of poetry, politics, and popular performance. In London, I had worked with the ANC in exile, even met with the hardworking Oliver Tambo, his legal partner, the one that held together a party then dubbed terrorist by the West. So Mandela’s name was 2. Parts of this article have been published in the Standard (Kenya); and the Sunday Inde- pendent (South Africa). boundary 2 41:2 (2014) DOI 10.1215/01903659-2686034 © 2014 Ngũgĩ wa Thiong’o Downloaded from http://read.dukeupress.edu/boundary-2/article-pdf/41/2/29/396837/b2412_12Thiongo_Fpp.pdf by guest on 23 September 2021 30 boundary 2 / Summer 2014 always in the horizon of my being, and now, at last, I was going to meet the man. -

First Published January 1884 February 2012, Vol
South African Medical Journal First published January 1884 February 2012, Vol. 102, No. 2 SAMJ WSU Medical School – a study in innovation and resilience That the Walter Sisulu University medical school continues to Didactic lectures were drastically reduced. Students accustomed to exist and to produce competent health professionals is evidence spoon-fed rote learning and fact memorisation now had to learn to of extraordinary institutional resilience. The school is located in a analyse and relate theoretical information to real-life situations, and to university with a troubled history. But even as the university has acquire knowledge through understanding and critical reasoning. In lurched from crisis to crisis and has periodically been under threat of terms of CBE, much of the clinical training occurred around district closure, the medical school has developed and grown to become the hospitals, rural clinics and community centres under the guidance of university’s premier centre of excellence and, it has often been said, family practitioners. The University-Community Health Partnership its raison d’etre, thanks in large part to sheer grit, commitment and Programme (UCHPP) brought the university, the community and sense of mission on the part of its leaders and academic community. ruling chiefs together to consult on local health needs and design Founded as a faculty of the University of Transkei in 1985, the interventions together in a true town-gown-crown partnership. The medical school began with little or no academic staff or teaching UCHPP, initially funded by the Kellogg Foundation, saw community facilities. Its development was like building a passenger airliner in health centres established by the university in remote rural sites. -

GENERAL PROSPECTUS 2021 General Rules and Regulations
WALTER SISULU UNIVERSITY GENERAL PROSPECTUS 2021 General Rules and Regulations @WalterSisuluUni Walter Sisulu University www.wsu.ac.za GENERAL PROSPECTUS 2021 This General Prospectus applies to all four campuses of Walter Sisulu University. LEGAL RULES 1. The University may in each year amend its rules. 2. The rules, including the amended rules, are indicated in the 2021 Prospectus. 3. The rules indicated in the 2021 Prospectus will apply to each student registered at Walter Sisulu University for 2021. 4. These rules will apply to each student, notwithstanding whether the student had first registered at the University prior to 2021. 5. When a student registers in 2021, the student accepts to be bound by the rules indicated in the 2021 prospectus. 6. The University may amend its rules after the General Prospectus has been printed. Should the University amend its rules during 2021, the amended rules will be communicated to students. Students will be bound by such amended rules. CAMPUSES & FACULTIES MTHATHA CAMPUS 1. Faculty of Commerce & Administration 2. Faculty of Educational Sciences 3. Faculty of Health Sciences 4. Faculty of Humanities, Social Sciences & Law 5. Faculty of Natural Sciences BUTTERWORTH CAMPUS 1. Faculty of Education 2. Faculty of Engineering & Technology 3. Faculty of Management Sciences BUFFALO CITY CAMPUS 1. Faculty of Business Sciences 2. Faculty of Science, Engineering & Technology KOMANI CAMPUS 1. Faculty of Economics & Information Technology Systems 2. Faculty of Education & School Development 1 2021PROSPECTUS -

Decolonising Research & Curatorial Practice In
UBUNTU MANIFEST: DECOLONISING RESEARCH & CURATORIAL PRACTICE IN CERAMICS. Wendy Anne Gers A thesis submitted in partial fulfilment of the requirements of the University of Sunderland for the degree of Doctor of Philosophy / PhD by Existing Published or Creative Works April 2019 Table of Contents PART 0 Abstract .................................................................................... 9 0.1 Technical notes .............................................................................. 10 0.1.1 Dedication & Acknowledgements ............................................ 10 0.1.2 Notes on style & referencing ................................................... 11 0.2 Glossary......................................................................................... 11 0.3 Introduction .................................................................................... 16 0.3.1 Overview ................................................................................. 16 0.3.2 Thesis structure ....................................................................... 17 PART 1 Scorched Earth ...................................................................... 19 1.1 Contextual overview: South African Art History from the 1990s to 2000s ................................................................................................ 20 1.2 Research methods ..................................................................... 22 1.2.1 Literature survey: Southern African & British commercial ceramics sectors ............................................................................. -

Redalyc.Student Opinions on Factors Influencing Tutorials at Walter
MEDICC Review ISSN: 1555-7960 [email protected] Medical Education Cooperation with Cuba Estados Unidos Garí, Mayra A.; Iputo, Jehu E. Student Opinions on Factors Influencing Tutorials at Walter Sisulu University, South Africa MEDICC Review, vol. 17, núm. 3, 2015, pp. 13-17 Medical Education Cooperation with Cuba Oakland, Estados Unidos Available in: http://www.redalyc.org/articulo.oa?id=437542102004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Original Research Student Opinions on Factors Influencing Tutorials at Walter Sisulu University, South Africa Mayra A. Garí MD and Jehu E. Iputo MBChB PhD ABSTRACT Later, in a joint session, 17 items previously identifi ed by both groups INTRODUCTION Problem-based learning harmonized with education were selected and included in a survey given to all 97 students at the in and for the community is the cornerstone of the curriculum for the end of second year. The survey assessed presence of each item in 0, undergraduate medical degree at Walter Sisulu University, Mthatha, 1, 2, 3 or 4 of the learning blocks. South Africa. In tutorials, students construct knowledge and learn to work collaboratively while interacting with one another in their search RESULTS Survey response was 93.8%. Mean reported presence of for solutions to a pedagogically modeled health issue based on a factors that infl uenced tutorials in the four learning blocks was 2.71 patient. Problems cover students’ needs defi ned by the learning cycle (SD 0.31) for the social dimension, 3.02 for motivational (SD 0.02), of the second year medical curriculum, organized into four learning 3.00 for cognitive (SD 0.42), and 2.22 for self-directed learning (SD blocks. -
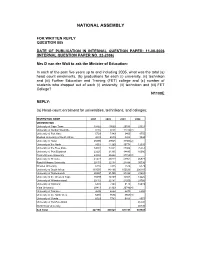
National Assembly
NATIONAL ASSEMBLY FOR WRITTEN REPLY QUESTION 885 DATE OF PUBLICATION IN INTERNAL QUESTION PAPER: 11-08-2006 (INTERNAL QUESTION PAPER NO. 22-2006) Mrs D van der Walt to ask the Minister of Education: In each of the past five years up to and including 2005, what was the total (a) head count enrolments, (b) graduations for each (i) university, (ii) technikon and (iii) Further Education and Training (FET) college and (c) number of students who dropped out of each (i) university, (ii) technikon and (iii) FET College? N1108E REPLY: (a) Head-count enrolment for universities, technikons, and colleges: INSTITUTION NAME 2001 2002 2003 2004 UNIVERSITIES University of Cape Town 18462 19560 20533 21321 University of Durban Westville 8155 9251 11270(2) University of Fort Hare 5729 7349 6405 8755 Medical University of South Africa 4039 4039 3883 3844 University of Natal 26090 29028 31925(2) University of the North 8551 11260 10774 12315 University of the Free State 14032 17451 21984 25351 University of Port Elizabeth 23225 21335 14485 16080 Potchefstroom University 23080 25442 27729(3) University of Pretoria 41473 40773 41951 46971 Rand Afrikaans University 20174 22134 24498 30736 Rhodes University 6216 7425 7526 6179 University of South Africa 133555 143136 150533 206187 University of Stellenbosch 20557 21395 21398 21451 University of the Western Cape 10499 12729 14043 14225 University of Witwatersrand 20132 22181 24250 24766 University of Zululand 6320 7393 9178 10419 Vista University 20413 21369 20746(4) University of Transkei 4605 4622 6479 6450 -

A Revolutionary Life Cut Short: Bathandwa Ndondo Memorial Lecture
A REVOLUTIONARY LIFE CUT SHORT: BATHANDWA NDONDO MEMORIAL LECTURE Adv. Sonwabile Mancotywa CEO, National Heritage Council SASCO Bathandwa Ndondo Memorial Lecture, Walter Sisulu University, 14 April 2016 Introduction Programme Director, Office bearers and members of SASCO, Walter Sisulu University management and staff, Honoured guests, Friends and comrades Thank you for the invitation to present this memorial lecture. As you all know, SASCO and this University occupy a very special place in my heart. Both the university and the person whom we are honouring tonight played a very formative role in my own political development. Cde Bathandwa Ndondo - ‘Bura’ as he was affectionately known - is a hero of our struggle for liberation whom I greatly admire and sacrifice must never be forgotten. I consider myself fortunate in that I followed in the second wave of activism pioneered by Bura and his comrades. I started studying law at UNITRA in the year of his murder. It is especially significant that this memorial lecture is being held during April – Freedom Month – as it is an appropriate time to pause and take stock of the road we have travelled and the challenges facing us as a nation. Let me therefore congratulate the organisers of this memorial lecture for their initiative in keeping alive the memory of Cde Bathandwa. Let me also congratulate the Bathandwa Ndondo Branch of SASCO at Walter Sisulu University. I am told that this branch was declared to be the biggest and most vibrant SASCO branch across the country by the 19th National General Congress. I am also pleased to see that 1 SASCO currently leads the SRC at this university.