Advincula Delineator™ Uterine Manipulator
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product Catalog Stainless Steel Vaginal Specula
PRODUCT CATALOG STAINLESS STEEL VAGINAL SPECULA Graves Speculum Product No. Description LTL-GS300 Graves Speculum, Small 3” x .75” LTL-GS400 Graves Speculum, Medium 4” x 1.5” LTL-GS450 Graves Speculum, Large 4.50” x 1.5” LTL-GS700 Graves Speculum, XL 7” x 1.5” Pederson Speculum Product No. Description LTL-PS305 Pederson Speculum, Virginal 3” x .5” LTL-PS300 Pederson Speculum, Small 3” x 1” LTL-PS400 Pederson Speculum, Medium 4” x 1” LTL-PS450 Pederson Speculum, Large 4.5” x 1” LTL-PS455 Pederson Speculum, Extra Narrow 4.5” x .5” LTL-PS700 Pederson Speculum, XL 7” x 1” Open Sided Speculum Product No. Description LTL-WGR400 Weisman-Graves Speculum, Medium, Right Open 4” x 1.5” LTL-WGR450 Weisman-Graves Speculum, Large, Right Open 4.5” x 1.5” LTL-WGL400 Weisman-Graves Speculum, Medium, Left Open 4” x 1.5” LTL-WGL450 Weisman-Graves Speculum, Large, Left Open 4.5” x 1.5” LTL-WPR400 Weisman-Pederson Speculum, Medium, Right Open 4” x 1” LTL-WPR450 Weisman-Pederson Speculum, Large, Right Open 4.5” x 1” LTL-WPL400 Weisman-Pederson Speculum, Medium, Left Open 4” x 1” LTL-WPL450 Weisman-Prderspm Speculum, Large, Left Open 4.5” x 1” *We also offer wide view (4cm) and full view (7cm) openings. 1 | TOLL FREE 1 [800] 910-8303 FAX 1 [805] 579-9415 WWW.LTLMEDICAL.NET BIOPSY PUNCHES Standard Style Rotating Style Tischler [Morgan] 7mm x 3mm Baby Tischler 4mm x 2mm Tischler Kevorkian 9.5mm x 3mm Product No. Description Product No. Description Product No. -

Inhaltsverzeichnis Index Index Indice Alfabético Indice
Inhaltsverzeichnis Index Index Indice alfabético Indice Inhaltsverzeichnis Index Index Indice alfabético Indice E-01 Inhaltsverzeichnis Index Index Indice alfabético Indice A B Accessories for sterilization container ......... 88-38 to 88-41 BABCOCK seizing forceps ........................................ 64-02 Adenotome LAFORCE .............................................. 46-19 BABINSKY percussion hammer ............................... 02-07 ADLERKREUTZ thumb and tissue forceps ............... 10-04 BACKHAUS-CLIP tube holder towel clamp .............. 14-03 ADSON BABY hemostatic forceps ............................ 12-09 BACKHAUS KOCHER towel clamp .......................... 14-02 ADSON-Baby retractor .............................................. 18-15 BACKHAUS towel clamp ........................................... 14-02 ADSON BAGGISH uterine biopsy specimen forceps ............. 70-45 bone rongeur ........................................................ 32-03 BAILEY-BABY rib contractor ..................................... 56-18 ADSON-BROWN thumb and tissue forceps ............. 10-03 BAILEY-GIBBON rib contractor ................................. 56-18 ADSON BAILEY rib contractor ............................................... 56-18 elevator ................................................................. 32-21 BAINBRIDGE hemostatic forceps ............................................... 12-09 atraumatic forceps ................................................ 13-09 hypophyseal forceps ............................................ -

AUA BLUS Handbook of Laparoscopic and Robotic Fundamentals
AUA BLUS Handbook of Laparoscopic and Robotic Fundamentals Sean Collins, Daniel S. Lehman, Elspeth M. McDougall, Ralph V. Clayman, and Jaime Landman ©American Urological Association Education & Research, Inc. Table of Contents 1. Introduction 2. Patient selection a. Indication b. contradindications c. special considerations 3. Physiologic effects of pneumoperitoneum a. Renal surgery transperitoneal b. Renal surgery retroperitoneal c. Hand-assisted laparoscopic nephrectomy d. Prostatectomy 4. Getting Started 5. Patient positioning a. Renal surgery transperitoneal b. Renal surgery retroperitoneal c. Hand-assisted laparoscopic nephrectomy d. Prostatectomy 6. Strategic placement of surgical team and operating room (OR) equipment 7. Access a. Primary access b. Renal surgery transperitoneal trocar placement c. Renal surgery retroperitoneal trocar placement d. Secondary access e. Retroperitoneal primary and secondary access f. Hand-assisted laparoscopic nephrectomy trocar placement g. Prostatectomy trocar placement 8. Instrumentation a. Trocars i. Cutting ii. Dilating iii. Radially dilating b. Bipolar cautery c. Monopolar cautery d. Ultrasonic instrumentation e. Vessel sealing devices i. LigaSure ii. Enseal f. Staplers g. Vascular clamps h. Suture anchors i. Titanium clips j. Locking clips q. Retractors r. Hemostatic agents s. Hand Assisted devices 2 9. Technique for Transperitoneal Laparoscopic Nephrectomy 10. Complications of laparoscopic surgery 3 Chapter 1. Introduction The American Urological Association (AUA) has prepared this handbook for all those new to laparoscopy. Rather than being a detailed surgical atlas, this is a handbook designed to introduce the fundamental principles of laparoscopy including: indications and contraindications for laparoscopy, the physiologic effects of pneumoperitoneum, patient positioning; abdominal access and trocar placement; strategic placement of the operating room (OR) team and equipment, overview of laparoscopic instrumentation, and complications unique to laparoscopic surgery. -

Fine Surgical Instruments for Research™
FINE SCIENCE TOOLS CATALOG 2021 FINE SCIENCE TOOLS CATALOG FINE SURGICAL INSTRUMENTS FOR RESEARCH™ TABLE OF CONTENTS | CATALOG 2021 Scissors 3 – 35 Spring 3 – 14 Fine 15 – 28 Letter from the Managing Partner Surgical 29 – 35 Bone Instruments 36 – 49 Rongeurs 36 – 38 Dear Customers, Cutters 39 – 47 Other Bone Instruments 47 – 48 After my uncle and founder of Fine Science Tools, Hans, handed Curettes & Chisels 49 over the management of the company to my cousin Rob and I Scalpels & Knives 50 – 61 last year, a lot has happened. Coming to FST as an “outsider”, my primary goal was to learn everything about the products, customers and the entire company. From my very first day, Forceps 63 – 91 I learned just how much my uncle Hans and the excellent Dumont 63 – 73 managerial team, Rob, Michael and Christina were able to Fine 72 – 80 grow the company over the last 45 years from a single office in Moria 74 S&T 75, 77 Vancouver into a global enterprise, a tremendous achievement Standard 81 – 91 that they should be proud of. Through excellent customer Hemostats 92 – 97 service, impeccable product quality and a passionate team, FST has become a household name in surgical and microsurgical instruments and accessories. During the COVID19 pandemic and the following worldwide lockdown, whether at their home office or on-site, our teams Probes & Hooks 99 – 103 around the world were able to provide our customers with the Spatulae 102 – 105 instruments and accessories they needed. Under challenging Hippocampal Tools & Spoons 106 – 107 circumstances, we kept our warehouses open in order to ship Pins & Holders 108 – 109 products to research laboratories, biotech’s and academic Wound Closure 110 – 121 institutions around the world while our support staff actively Needle Holders & Suture 110 – 118 Staplers, Clips & Applicators 119 – 121 continued to provide assistance to all customer questions and Retractors 122 – 129 inquiries. -

Outpatient Surgical Procedures – Site of Service: CPT/HCPCS Codes
UnitedHealthcare® Commercial Policy Appendix: Applicable Code List Outpatient Surgical Procedures – Site of Service: CPT/HCPCS Codes This list of codes applies to the Utilization Review Guideline titled Effective Date: August 1, 2021 Outpatient Surgical Procedures – Site of Service. Applicable Codes The following list(s) of procedure and/or diagnosis codes is provided for reference purposes only and may not be all inclusive. The listing of a code does not imply that the service described by the code is a covered or non-covered health service. Benefit coverage for health services is determined by the member specific benefit plan document and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claim payment. Other Policies and Guidelines may apply. This list contains CPT/HCPCS codes for the following: • Auditory System • Female Genital System • Musculoskeletal System • Cardiovascular System • Hemic and Lymphatic Systems • Nervous System • Digestive System • Integumentary System • Respiratory System • Eye/Ocular Adnexa System • Male Genital System • Urinary System CPT Code Description Auditory System 69100 Biopsy external ear 69110 Excision external ear; partial, simple repair 69140 Excision exostosis(es), external auditory canal 69145 Excision soft tissue lesion, external auditory canal 69205 Removal foreign body from external auditory canal; with general anesthesia 69222 Debridement, mastoidectomy cavity, complex (e.g., with anesthesia or more -

Surgical Technique Guide Description
Surgical Technique Guide Description The PrimaLIF™ LIFF Interbody Fusion System is a PEEK interbody cage system implanted to replace collapsed, damaged, or unstable vertebral discs due to degenerative conditions, tumor or trauma. The system is designed to provide anterior spinal column support and bone graft containment to promote bony fusion. This Lateral Lumbar Interbody Fusion (LLIF) system includes insertion instruments for the interbody implant, a retractor system, table arm, and disc preparation instruments. Indications for Use The OsteoMed Spine PrimaLIF™ LLIF is indicated for intervertebral body fusion of the lumbar spine to be used with autogenous bone graft, from L2 to S1, in skeletally mature patients who have had six months of non-operative treatment. The device is intended for use at either one level or two contiguous levels for the treatment of degenerative disc disease (DDD) and these patients may have up to Grade I spondylolisthesis or retrolisthesis at the involved levels. DDD is defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies. The device is intended for use with supplemental fixation such as PrimaLOK™ SP and PrimaLOK™ FF which has been cleared for use in the lumbar spine. For additional information, warnings and contraindications, please refer to the Product Insert. TABLE OF CONTENTS Product Labeling Description Inside Indications for Use Cover Design Rationale Rethinking Lateral Access 1 Retractor Highlights 1 Interbody Implant Highlights 2 Surgical -
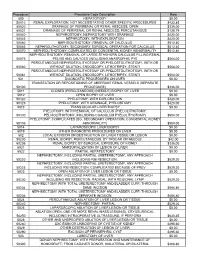
Procedure Procedure Code Description Rate 500
Procedure Procedure Code Description Rate 500 HEPATOTOMY $0.00 50010 RENAL EXPLORATION, NOT NECESSITATING OTHER SPECIFIC PROCEDURES $433.85 50020 DRAINAGE OF PERIRENAL OR RENAL ABSCESS; OPEN $336.00 50021 DRAINAGE OF PERIRENAL OR RENAL ABSCESS; PERCUTANIOUS $128.79 50040 NEPHROSTOMY, NEPHROTOMY WITH DRAINAGE $420.00 50045 NEPHROTOMY, WITH EXPLORATION $420.00 50060 NEPHROLITHOTOMY; REMOVAL OF CALCULUS $512.40 50065 NEPHROLITHOTOMY; SECONDARY SURGICAL OPERATION FOR CALCULUS $512.40 50070 NEPHROLITHOTOMY; COMPLICATED BY CONGENITAL KIDNEY ABNORMALITY $512.40 NEPHROLITHOTOMY; REMOVAL OF LARGE STAGHORN CALCULUS FILLING RENAL 50075 PELVIS AND CALYCES (INCLUDING ANATROPHIC PYE $504.00 PERCUTANEOUS NEPHROSTOLITHOTOMY OR PYELOSTOLITHOTOMY, WITH OR 50080 WITHOUT DILATION, ENDOSCOPY, LITHOTRIPSY, STENTI $504.00 PERCUTANEOUS NEPHROSTOLITHOTOMY OR PYELOSTOLITHOTOMY, WITH OR 50081 WITHOUT DILATION, ENDOSCOPY, LITHOTRIPSY, STENTI $504.00 501 DIAGNOSTIC PROCEDURES ON LIVER $0.00 TRANSECTION OR REPOSITIONING OF ABERRANT RENAL VESSELS (SEPARATE 50100 PROCEDURE) $336.00 5011 CLOSED (PERCUTANEOUS) (NEEDLE) BIOPSY OF LIVER $0.00 5012 OPEN BIOPSY OF LIVER $0.00 50120 PYELOTOMY; WITH EXPLORATION $420.00 50125 PYELOTOMY; WITH DRAINAGE, PYELOSTOMY $420.00 5013 TRANSJUGULAR LIVER BIOPSY $0.00 PYELOTOMY; WITH REMOVAL OF CALCULUS (PYELOLITHOTOMY, 50130 PELVIOLITHOTOMY, INCLUDING COAGULUM PYELOLITHOTOMY) $504.00 PYELOTOMY; COMPLICATED (EG, SECONDARY OPERATION, CONGENITAL KIDNEY 50135 ABNORMALITY) $504.00 5014 LAPAROSCOPIC LIVER BIOPSY $0.00 5019 OTHER DIAGNOSTIC PROCEDURES -

DILAPAN-S® Hygroscopic Cervical Dilator Instructions For
DILAPAN-S® Hygroscopic Cervical Dilator Re-use / re-sterilization / reprocessing1) of the DILAPAN-S® single-use medical device may result in physical damage to the medical device, failure of intended use of the medical device, and illness or injury to the patient as a result of infection, inflammation Instructions for Use and / or disease due to product contamination, infections and insufficient sterility of the product. GENERAL INFORMATION 1) A process carried out on a used device in order to allow its safe reuse including cleaning. Content Careful placement of the device is essential to avoid traumatic injury to the cervix or A sterile hygroscopic cervical dilator packed in a printed composite primary peel-open uterus and to avoid migration of the device either upward into the uterus or downward pouch, a piece of Instructions for use. into the vagina. ® The DILAPAN-S® is available in a box of 25 dilators and in the following dimensions: The DILAPAN-S may fragment during removal using incorrect technique. Fragmentation 4×65 mm, 4×55 mm, 3×55 mm. may result in pieces of the device being retained in the uterus. Carefully follow the Removal instructions. Device description and performance Do not use if primary packaging has been opened or damaged. Synthetic hydrogel cervical dilator consists of the dilating part, the polypropylene handle and the marker string (see the figure below). The dilating part is manufactured from Do not re-use, intended for one-time use. an anisotropic xerogel of AQUACRYL. The dilator is capable of increasing in diameter as it absorbs moisture from the genital tract. -

Federal Chargemaster Price Transparency Edgewood (2).Xlsx
EDGEWOOD SURGICAL HOSPITAL CHARGES Federal reporting rules require hospitals to maintain a catalog of thousands of procedure codes, code descriptions and list prices in a complex accounting tool, known as the hospital chargemaster. The prices listed on the chargemaster do not reflect what patients will ultimately pay as insurance companies negotiate discounts on the list prices. In addition, co-pays, co-insurance and deductibles can also bring additional discounts before a final charge is determined. To get an accurate estimate of what your out of pocket expenses will be, contact us at (724) 646-0400, Monday through Friday, from 8 a.m. – 4:30 p.m. Chg Code Description Chg Amt 1 NF-HUMULIN R INJ SOLN 100U/1ML $61.61 99077 EXTENDED RECOVERY ROOM PER MINUTE $15.00 99078 OBSERVATION 1-4 HOURS $550.00 99079 OBSERVATION >5 HOURS **EACH** $15.00 99085 OR TIME PER MINUTE COMPLEX (>3 STAFF) $197.00 99086 OR TIME PER MINUTE MAJOR (3 STAFF) $136.00 99087 OR TIME PER MINUTE MINOR (1-2 STAFF) $93.00 99088 SURGICAL NEUROMONITORING $1,350.00 99089 SURGICAL EYE LASER $1,743.00 99090 PAIN MANAGEMENT PER MINUTE $187.00 99091 OR TIME PER MINUTE ADDITIONAL STAFF $1.00 99092 FORCE TRIAD RENTAL $350.00 99093 YAG LASER CHARGE $1,182.00 99094 PAIN MANAGEMENT PER MINUTE RF $326.00 99100 CONS SEDATION (SAME DOC) <5YR 30-MIN $302.00 99101 CONS SEDATION (SAME DOC) <5YR 30-MIN $302.00 99102 CONS SEDATION (SAME DOC) ADD'S 15-MIN $151.00 99103 CONS SEDATION (DIFF DOC) <5YR 30-MIN $302.00 99104 CONS SEDATION (DIFF DOC) >5 YR 30-MIN $302.00 99105 CONS SEDATION (DIFF -

Product Catalog
precision crafted quality PRODUCT CATALOG www.medicaldevicepurchase.com 1-916-663-4165 2 precision crafted quality A letter from our CEO: Medical Device Purchase is a company started by my father and me almost 10 years ago. We knew the road ahead for MDP would be challenging and that we would inevitably face giant corporations that had been established in the industry for decades. Many said we would not even last two years. However, our approach was different from our competitors. We wanted to create a friendlier environment for healthcare professionals searching for industry-leading surgical products. How? By providing more—and higher quality—options. Our mission was to provide a welcoming environment where customers could enjoy a uniquely approachable buying experience. We wanted to build a brand that focused on creating efficiency and reliability so that our clients could spend more time on the things that really matter. Dr. Ray, our co-founder, passed away earlier this year, but the legacy of Medical Device Purchase lives on as we continue to grow and expand as a cutting edge company blazing new trails in the world of surgical products. Sincerely, Orin Ray www.medicaldevicepurchase.com 3 About MDP WHO WE ARE AND WHAT WE DO Medical Device Purchase is a leading supplier of premium quality surgical products, committed to satisfying the ever-growing demands of the healthcare community. We provide a new level of reliability, efficiency, and value by using applications, performance products, and technology unlike any any other supplier in the the industry. OUR MISSION As the cost of healthcare continues to rise, MDP remain committed to reducing your overhead. -

Chirurgische Instrumente Surgical Instruments
CHIRURGISCHE INSTRUMENTE SURGICAL INSTRUMENTS SURGICAL INSTRUMENTS Percussion Hammers & Aesthesiometers 01-103 01-102 DEJERINE 01-104 DEJERINE With Needle TAYLOR Size: 200 mm Size: 210 mm Size: 195 mm 01-101 ½ ½ ½ TROEMNER Size: 245 mm ½ 01-109 01-106 01-107 WARTENBERG BUCK RABINER Pinwheal For 01-105 With Needle With Needle 01-108 Neurological BERLINER And Brush And Brush ALY Examination Size: 200 mm Size: 180 mm Size: 255 mm Size: 190 mm Size: 185 mm ½ ½ ½ ½ ½ Page 1 2 Stethoscopes 01-112 01-110 01-111 BOWLES PINARD (Aluminum) aus Holz (Wooden) Stethoscope Size: 155 mm Size: 145 mm With Diaphragm ½ ½ 01-113 01-114 ANESTOPHON FORD-BOWLES Duel Chest Piece 01-115 With Two Outlets BOWLES Page 2 3 Head Mirrors & Head Bands 01-116 01-117 ZIEGLER mm ZIEGLER mm Head mirror only Head mirror only with rubber coating with metal coating 01-118 01-120 ZIEGLER MURPHY Head band of plastic black Head band of celluloid, white 01-119 ZIEGLER Head band of plastic white 01-121 01-122 Head band of plastic, Head mirror with black white, soft pattern plastic head band. Page 3 4 Head Light 01-123 CLAR Head light, 6 volt, with adjustable joint, white celluloid head band, cord with plugs for transformer 01-124 White celluloid head band, only, for 01-125 Spare mirror only, for 01-126 spare bulb 01-127 CLAR Head light, 6 volt, with adjustable joint, white celluloid head band, with foam rubber pad and cord with plugs for transformer 01-128 White celluloid head band, only, for head light 01-129 mirror only, for head light 01-130 spare foam rubber pad, for head band -

Cardiovascular/ Thoracic Portfolio
CVT-2 CARDIOVASCULAR/THORACIC Cardiovascular/ Scan the code above to learn more about our Cardiovascular/ Thoracic portfolio. Thoracic Portfolio Questions? Contact us at [email protected] or learn more at symmetrysurgical.com CARDIOVASCULAR/THORACIC CVT-1 Cardiovascular/Thoracic CVT Approximators CVT-2 Punches CVT-136 Cannulas CVT-3 Raspatory CVT-137 Clamps CVT-3 Retractors CVT-138 Containers CVT-55 Rib Spreader CVT-186 Cords CVT-63 Rongeurs CVT-187 Cups CVT-63 Saws CVT-188 Dilators CVT-64 Scissors CVT-188 Dissectors CVT-68 Shears CVT-216 Elevators CVT-70 Spatulas CVT-218 Forceps CVT-71 Spreaders CVT-218 Hooks CVT-121 Stringers CVT-220 Knife Handles CVT-121 Strippers CVT-222 Knives CVT-122 Suction Tubes CVT-223 Loops CVT-122 Sump Suction CVT-225 Markers CVT-122 Tourniquets CVT-225 Needle Holders CVT-123 Trocars CVT-225 Needles CVT-135 Tunnelers CVT-226 Obturators CVT-135 Valvulotomes CVT-227 Pliers CVT-135 Wire & Pin Cutters CVT-227 Questions? Contact us at [email protected] or learn more at symmetrysurgical.com Questions? Contact us at [email protected] or learn more at symmetrysurgical.com The Surgical Instrument Desk Reference How to Use Our Catalog U.S. Customer Service The Symmetry Surgical catalog is designed with Your Symmetry Surgical Customer Service team wants you in mind, to be your trusted resource for surgical to make ordering surgical instruments the easiest part instrumentation. We took great care in creating an of your day. We accept orders via phone, fax, email, our easy-to-use, robust catalog and we think you'll be website, Global Healthcare Exchange (GHX) and pleased with the design as you become more familiar through the mail.