Protocol for Provision of Intrauterine Contraception
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product Catalog Stainless Steel Vaginal Specula
PRODUCT CATALOG STAINLESS STEEL VAGINAL SPECULA Graves Speculum Product No. Description LTL-GS300 Graves Speculum, Small 3” x .75” LTL-GS400 Graves Speculum, Medium 4” x 1.5” LTL-GS450 Graves Speculum, Large 4.50” x 1.5” LTL-GS700 Graves Speculum, XL 7” x 1.5” Pederson Speculum Product No. Description LTL-PS305 Pederson Speculum, Virginal 3” x .5” LTL-PS300 Pederson Speculum, Small 3” x 1” LTL-PS400 Pederson Speculum, Medium 4” x 1” LTL-PS450 Pederson Speculum, Large 4.5” x 1” LTL-PS455 Pederson Speculum, Extra Narrow 4.5” x .5” LTL-PS700 Pederson Speculum, XL 7” x 1” Open Sided Speculum Product No. Description LTL-WGR400 Weisman-Graves Speculum, Medium, Right Open 4” x 1.5” LTL-WGR450 Weisman-Graves Speculum, Large, Right Open 4.5” x 1.5” LTL-WGL400 Weisman-Graves Speculum, Medium, Left Open 4” x 1.5” LTL-WGL450 Weisman-Graves Speculum, Large, Left Open 4.5” x 1.5” LTL-WPR400 Weisman-Pederson Speculum, Medium, Right Open 4” x 1” LTL-WPR450 Weisman-Pederson Speculum, Large, Right Open 4.5” x 1” LTL-WPL400 Weisman-Pederson Speculum, Medium, Left Open 4” x 1” LTL-WPL450 Weisman-Prderspm Speculum, Large, Left Open 4.5” x 1” *We also offer wide view (4cm) and full view (7cm) openings. 1 | TOLL FREE 1 [800] 910-8303 FAX 1 [805] 579-9415 WWW.LTLMEDICAL.NET BIOPSY PUNCHES Standard Style Rotating Style Tischler [Morgan] 7mm x 3mm Baby Tischler 4mm x 2mm Tischler Kevorkian 9.5mm x 3mm Product No. Description Product No. Description Product No. -

Catalogue of Surgical Instruments, for Sale by Codman & Shurtleff, 13
CATALOGUE OF jittigical KttjgtrttittitttjGi, FOR SALE BY CODMAI & SHUETLEPF, 13, Tremont Street, Boston. Amputating Case, containing the following warranted instruments of first quality and finish, in handsome brass-hound Rosewood Case, 16 inches long, 4\ wide, and high: — Capital Saw, Metacarpal Saw, Bone Forceps, Spring-catch Artery Forceps, four Amputating Knives, Tenaculum, Tourniquet, half-dozen assorted Needles, and Ligature Silk, . $25.00 Amputating Case, Mahogany, 16 inches long, 4\ wide, 8j high, containing the following instruments, of plainer finish than the above, first quality, war- ranted : — Capital Saw, Metacarpal Saw, four Am- putating Knives, Bone Forceps, Slide-catch Artery Forceps, Tenaculum, Tourniquet, four assorted Needles, and Ligature Silk 20.00 Amputating Case, Mahogany, inches long, 6 wide, 2f high, containing: — Capital Saw, Metacarpal Saw, three Amputating Knives, large Scalpel, Spring-catch Artery Forceps, Bone Forceps, Tena- culum, Tourniquet, one dozen assorted Needles, and Ligature Silk 18.50 2 CODMAN AND SHURTLEFF’S Amputating and Trepanning Case, Rosewood, brass bound, 16 inches long, wide, 3 high, containing the following instruments of first quality and finish, warranted:— Capital Saw, Metacarpal Saw, Bone Forceps, Spring-catch Artery Forceps, three Amputating Knives, large Scalpel, Tenaculum, Tourniquet, half-dozen assorted Needles, two Tre- phines, Hey’s Saw, Elevator, Brush, and Ligature Silk $35.00 Amputating and Trepanning Case (Parker’s Com- pact), Rosewood, brass bound, 12 inches long, 4 wide, 2J high, containing the following ivory- mounted instruments of best quality and finish, warranted:— Capital Saw, Metacarpal Saw, Hey’s Saw, three Amputating Knives adapted to one handle by screw, Finger Knife, Spring-catch Artery Forceps, Bone Forceps, Tenaculum, Tourniquet, Trephine, Elevator, Brush, six assorted Needles, and Ligature Silk 35.00 Amputating Cases fitted up to order, at prices corres- ponding with number and style of instruments. -

Model for Teaching Cervical Dilation and Uterine Curettage
Model for Teaching Cervical Dilation and Uterine Curettage Linda J. Gromko, MD, and Sam C. Eggertsen, MD Seattle, W a s h in g to n t least 15 percent of clinically recognizable pregnan METHODS A cies terminate in fetal loss, with the majority occur ring in the first trimester.1 Cervical dilation and uterine The fabric model was developed under the guidance of curettage (D&C) is frequently important in the manage physicians at the University of Washington Department ment of early pregnancy loss to control bleeding and re of Family Medicine and is commercially available.* The duce the risk of infection. D&Cs are also done for thera model, designed to approximate a 10-week last-menstrual- peutic first trimester abortions in family practice settings. period-sized uterus, is supported by elastic “ligaments” Resident experience may vary greatly, and some may feel on a wooden frame (Figure 1). A standard Graves spec inadequately trained in this procedure. The initial use of ulum can be inserted into the “vagina,” permitting vi gynecologic instruments (ie, tenaculum, sound, dilators, sualization of a cloth cervix. After placement of a tena curette) can feel awkward to the learner, and extensive culum onto the cervix, a paracervical block can be verbal tutoring may be discomfiting to the awake patient. demonstrated and the uterus sounded. Progressive dilation Training on a model can reduce these problems. After with Pratt or Denniston dilators follows: a drawstring al gaining basic skills on a model, the resident can focus on lows for the cervix to retain each successive degree of di gaining additional skills and refining technique during pa lation. -
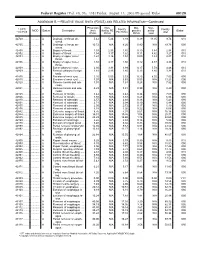
RELATIVE VALUE UNITS (RVUS) and RELATED INFORMATION—Continued
Federal Register / Vol. 68, No. 158 / Friday, August 15, 2003 / Proposed Rules 49129 ADDENDUM B.—RELATIVE VALUE UNITS (RVUS) AND RELATED INFORMATION—Continued Physician Non- Mal- Non- 1 CPT/ Facility Facility 2 MOD Status Description work facility PE practice acility Global HCPCS RVUs RVUs PE RVUs RVUs total total 42720 ....... ........... A Drainage of throat ab- 5.42 5.24 3.93 0.39 11.05 9.74 010 scess. 42725 ....... ........... A Drainage of throat ab- 10.72 N/A 8.26 0.80 N/A 19.78 090 scess. 42800 ....... ........... A Biopsy of throat ................ 1.39 2.35 1.45 0.10 3.84 2.94 010 42802 ....... ........... A Biopsy of throat ................ 1.54 3.17 1.62 0.11 4.82 3.27 010 42804 ....... ........... A Biopsy of upper nose/ 1.24 3.16 1.54 0.09 4.49 2.87 010 throat. 42806 ....... ........... A Biopsy of upper nose/ 1.58 3.17 1.66 0.12 4.87 3.36 010 throat. 42808 ....... ........... A Excise pharynx lesion ...... 2.30 3.31 1.99 0.17 5.78 4.46 010 42809 ....... ........... A Remove pharynx foreign 1.81 2.46 1.40 0.13 4.40 3.34 010 body. 42810 ....... ........... A Excision of neck cyst ........ 3.25 5.05 3.53 0.25 8.55 7.03 090 42815 ....... ........... A Excision of neck cyst ........ 7.07 N/A 5.63 0.53 N/A 13.23 090 42820 ....... ........... A Remove tonsils and ade- 3.91 N/A 3.63 0.28 N/A 7.82 090 noids. -

Vantage by Integra® Miltex® Surgical Instruments
Vantage® by Integra® Miltex® Surgical Instruments Table of Contents Operating Scissors ................................................................................................................................. 4 Scissors ................................................................................................................................................ 5-6 Bandage Scissors .................................................................................................................................... 7 Dressing and Tissue Forceps ................................................................................................................. 8 Splinter Forceps ...................................................................................................................................... 9 Hemostatic Forceps......................................................................................................................... 10-12 of Contents Table Towel Clamps ......................................................................................................................................... 13 Tubing Forceps .......................................................................................................................................14 Sponge and Dressing Forceps ............................................................................................................. 15 Needle Holders .................................................................................................................................16-17 -

Inhaltsverzeichnis Index Index Indice Alfabético Indice
Inhaltsverzeichnis Index Index Indice alfabético Indice Inhaltsverzeichnis Index Index Indice alfabético Indice E-01 Inhaltsverzeichnis Index Index Indice alfabético Indice A B Accessories for sterilization container ......... 88-38 to 88-41 BABCOCK seizing forceps ........................................ 64-02 Adenotome LAFORCE .............................................. 46-19 BABINSKY percussion hammer ............................... 02-07 ADLERKREUTZ thumb and tissue forceps ............... 10-04 BACKHAUS-CLIP tube holder towel clamp .............. 14-03 ADSON BABY hemostatic forceps ............................ 12-09 BACKHAUS KOCHER towel clamp .......................... 14-02 ADSON-Baby retractor .............................................. 18-15 BACKHAUS towel clamp ........................................... 14-02 ADSON BAGGISH uterine biopsy specimen forceps ............. 70-45 bone rongeur ........................................................ 32-03 BAILEY-BABY rib contractor ..................................... 56-18 ADSON-BROWN thumb and tissue forceps ............. 10-03 BAILEY-GIBBON rib contractor ................................. 56-18 ADSON BAILEY rib contractor ............................................... 56-18 elevator ................................................................. 32-21 BAINBRIDGE hemostatic forceps ............................................... 12-09 atraumatic forceps ................................................ 13-09 hypophyseal forceps ............................................ -

STILLE Surgical Instruments Kirurgisk Perfektion I Närmare 180 År Surgical Perfection for Almost 180 Years
STILLE Surgical Instruments Kirurgisk perfektion i närmare 180 år Surgical Perfection for almost 180 years I närmare 180 år har vi utvecklat och tillverkat de bästa kirurgiska For almost 180 years, we have developed and manufactured the best instrumenten till världens mest krävande kirurger. Vi vill rikta ett stort surgical instruments for the world’s most demanding surgeons. tack till alla våra trogna kunder och samtidigt välkomna våra nya kunder. We would like to extend a heartfelt thank you to all our loyal I den här katalogen presenterar vi vårt kompletta sortiment av customers and a warm welcome to our new customers. In this catalog STILLEs original instrument. we present our complete range of STILLE original surgical instruments. Precision, hållbarhet och känsla är typiska egenskaper för alla Precision, durability and feel are characteristic qualities of all STILLE STILLE-instrument. Den stora majoriteten är handgjorda av våra instruments. The vast majority are handcrafted by our highly skilled skickliga instrumentmakare Eskilstuna. Instrumentets resa från rundstål instrument makers in Eskilstuna, west of Stockholm, Sweden. The instru- till ett färdigt instrument är lång, och består av många tillverkningssteg. ments’ journey from a rod of stainless steel to a finished instrument is a STILLEs unika tillverkningsmetod och användning av enbart det bästa long one, involving multiple stages. STILLE’s unique method of crafting its materialet ger våra instrument deras unika känsla och hållbarhet. instrument materials, and its usage of only the very highest-grade steels, give our instruments their unique feel and durability. I det första kapitlet hittar du våra saxar, allt från vanliga operationssaxar till våra unika SuperCut och Diamond SuperCut-saxar. -

AUA BLUS Handbook of Laparoscopic and Robotic Fundamentals
AUA BLUS Handbook of Laparoscopic and Robotic Fundamentals Sean Collins, Daniel S. Lehman, Elspeth M. McDougall, Ralph V. Clayman, and Jaime Landman ©American Urological Association Education & Research, Inc. Table of Contents 1. Introduction 2. Patient selection a. Indication b. contradindications c. special considerations 3. Physiologic effects of pneumoperitoneum a. Renal surgery transperitoneal b. Renal surgery retroperitoneal c. Hand-assisted laparoscopic nephrectomy d. Prostatectomy 4. Getting Started 5. Patient positioning a. Renal surgery transperitoneal b. Renal surgery retroperitoneal c. Hand-assisted laparoscopic nephrectomy d. Prostatectomy 6. Strategic placement of surgical team and operating room (OR) equipment 7. Access a. Primary access b. Renal surgery transperitoneal trocar placement c. Renal surgery retroperitoneal trocar placement d. Secondary access e. Retroperitoneal primary and secondary access f. Hand-assisted laparoscopic nephrectomy trocar placement g. Prostatectomy trocar placement 8. Instrumentation a. Trocars i. Cutting ii. Dilating iii. Radially dilating b. Bipolar cautery c. Monopolar cautery d. Ultrasonic instrumentation e. Vessel sealing devices i. LigaSure ii. Enseal f. Staplers g. Vascular clamps h. Suture anchors i. Titanium clips j. Locking clips q. Retractors r. Hemostatic agents s. Hand Assisted devices 2 9. Technique for Transperitoneal Laparoscopic Nephrectomy 10. Complications of laparoscopic surgery 3 Chapter 1. Introduction The American Urological Association (AUA) has prepared this handbook for all those new to laparoscopy. Rather than being a detailed surgical atlas, this is a handbook designed to introduce the fundamental principles of laparoscopy including: indications and contraindications for laparoscopy, the physiologic effects of pneumoperitoneum, patient positioning; abdominal access and trocar placement; strategic placement of the operating room (OR) team and equipment, overview of laparoscopic instrumentation, and complications unique to laparoscopic surgery. -

Endo Product Guide 2018
Visualization Plastic and Reconstructive Surgery Arthroscopy Urology Gynecology Laparoscopy Services U.S. Product Guide 2018 Visualization Plastic and Arthroscopy 12 Camera systems and Reconstructive 40 Arthroscopes minimally invasive surgery Surgery 44 Arthroscope hardware 19 Open visualization 34 SPY-PHI Fluorescence 50 Powered instruments 20 Video enhancement Imaging System 53 Cutters & burs 21 Light source 35 SPY Elite Fluorescence Imaging System 59 Arthroscopy pumps 24 Data management 36 DermACELL Acellular 61 Single portal arthroscopy 27 Printers Dermal Matrix (ADM) 63 Hip arthroscopy 28 Flat panel surgical display 69 Small-joint arthroscopy 30 Wireless technology 71 Manual instruments 31 Video carts 74 Disposable cannulas 75 Micro cart Urology Laparoscopy Services 78 Rigid cystoscopes 96 Laparoscopes 116 OnSite Services 80 Resection 99 Manual instruments 117 ProCare Services 82 Laser cystoscope 106 Electrosurgical instruments 118 Replacement parts 83 Urethrotome 107 Suction Irrigation 120 Repair process 84 Ureteroscopes 110 Insuffators 121 Loaner and replacement policy 85 Urology accessories 112 ICG and infrared technology 113 Laparoscopic Gynecology training system Index 88 Rigid hysteroscopes 113 Pneumatic scope holder 122 Index 90 Resection 93 Gynecologic laparoscopy instruments 93 Fluid management We are This mission is at the heart of what we do and believe. We collaborate with our customers to develop innovative products and services that ultimately improve the lives of patients. We understand that in today’s challenging healthcare environment, our customers need solutions that Stryker improve quality and effciency in the operating room and, ultimately, the patient experience. These challenges are creating opportunities for us to Together with our customers, make healthcare better, for our customers and the we are driven to make healthcare better. -

Answer Key Chapter 1
Instructor's Guide AC210610: Basic CPT/HCPCS Exercises Page 1 of 101 Answer Key Chapter 1 Introduction to Clinical Coding 1.1: Self-Assessment Exercise 1. The patient is seen as an outpatient for a bilateral mammogram. CPT Code: 77055-50 Note that the description for code 77055 is for a unilateral (one side) mammogram. 77056 is the correct code for a bilateral mammogram. Use of modifier -50 for bilateral is not appropriate when CPT code descriptions differentiate between unilateral and bilateral. 2. Physician performs a closed manipulation of a medial malleolus fracture—left ankle. CPT Code: 27766-LT The code represents an open treatment of the fracture, but the physician performed a closed manipulation. Correct code: 27762-LT 3. Surgeon performs a cystourethroscopy with dilation of a urethral stricture. CPT Code: 52341 The documentation states that it was a urethral stricture, but the CPT code identifies treatment of ureteral stricture. Correct code: 52281 4. The operative report states that the physician performed Strabismus surgery, requiring resection of the medial rectus muscle. CPT Code: 67314 The CPT code selection is for resection of one vertical muscle, but the medial rectus muscle is horizontal. Correct code: 67311 5. The chiropractor documents that he performed osteopathic manipulation on the neck and back (lumbar/thoracic). CPT Code: 98925 Note in the paragraph before code 98925, the body regions are identified. The neck would be the cervical region; the thoracic and lumbar regions are identified separately. Therefore, three body regions are identified. Correct code: 98926 Instructor's Guide AC210610: Basic CPT/HCPCS Exercises Page 2 of 101 6. -

2019 Charge Schedules 12.20.2018.Xlsx
DESCRIPTION 2019 Charge HCHG =E GRADIENT STRIP 39.70 HCHG 10 ML ABLAVAR PER ML 37.10 HCHG 10 ML EOVIST PER ML 39.80 HCHG 11 - DEOXYCORTISOL 135.30 HCHG 14 3 3 PROTEIN CSF 347.00 HCHG 15 ML ABLAVAR PER ML 37.10 HCHG 17 OH PREGNENOLONE 87.70 HCHG 17/OH PROGESTERONE 66.20 HCHG 21 HYDROXYLASE ANTIBODY 297.30 HCHG 2TS AFP 139.60 HCHG 2TS ESTRIOL 197.80 HCHG 2TS HCG 95.20 HCHG 2TS INHIBIN A 130.00 HCHG 3D SENSOR VEST PR4270 12,818.00 HCHG 5HIAA QUANT 99.50 HCHG A1A PHENOTYPE 82.40 HCHG ABD PILLOW PR20 75.00 HCHG ABD RETRACTION SYSTEM 98.20 HCHG ABDOMINAL DOPPLER LTD STUDY 453.40 HCHG ABO GROUP 36.30 HCHG ABY SCREEN 97.90 HCHG ACCESS VENOUS ADVANCED 280.90 HCHG ACETAMINOPHEN 153.80 HCHG ACETYLCHOLINE RECEP BIND 180.00 HCHG ACETYLCHOLINE RECEPTOR MOD ABY 247.50 HCHG ACETYLCHOLINESTERASE RBC 147.70 HCHG ACHR GANGLIONIC NEURONAL ABY SERUM 264.00 HCHG ACT. PROTEIN C RESIST 158.60 HCHG ACTH 53.50 HCHG ACTH STIMULATION PANEL 182.00 HCHG ACTIVATED PTT 55.00 HCHG ACTIVE CORD 298.10 HCHG ACUTE HEPATITIS PANEL 239.50 HCHG ACYLCARNITINES QUANT PLASMA 150.50 HCHG ACYLGLYCINES,QUANT,URINE 484.80 HCHG ADAPTOR/EXT PACING/NEUROSTIMULATOR LEAD PR527 1,663.50 HCHG ADDL DISC SUSCEPT 58.00 HCHG ADENOVIRUS IGG 267.10 HCHG ADENOVIRUS PCR 245.70 HCHG ADHESION BARRIER PR250 787.50 HCHG ADHESION BARRIER PR275 862.50 HCHG ADHESION BARRIER PR300 945.00 HCHG ADHESION BARRIER PR330 1,038.00 HCHG ADMIN OF PNEUMOCOCCAL VACCINE 16.30 HCHG ADMIN OF SEASONAL INFLUENZA VACCINE 16.30 HCHG ADMIN SET PEDS FILTER & SYRINGE 29.30 HCHG ADMIN SET-PLTS & CRYO 291.20 HCHG ADMINISTRATION -

Fine Surgical Instruments for Research™
FINE SCIENCE TOOLS CATALOG 2021 FINE SCIENCE TOOLS CATALOG FINE SURGICAL INSTRUMENTS FOR RESEARCH™ TABLE OF CONTENTS | CATALOG 2021 Scissors 3 – 35 Spring 3 – 14 Fine 15 – 28 Letter from the Managing Partner Surgical 29 – 35 Bone Instruments 36 – 49 Rongeurs 36 – 38 Dear Customers, Cutters 39 – 47 Other Bone Instruments 47 – 48 After my uncle and founder of Fine Science Tools, Hans, handed Curettes & Chisels 49 over the management of the company to my cousin Rob and I Scalpels & Knives 50 – 61 last year, a lot has happened. Coming to FST as an “outsider”, my primary goal was to learn everything about the products, customers and the entire company. From my very first day, Forceps 63 – 91 I learned just how much my uncle Hans and the excellent Dumont 63 – 73 managerial team, Rob, Michael and Christina were able to Fine 72 – 80 grow the company over the last 45 years from a single office in Moria 74 S&T 75, 77 Vancouver into a global enterprise, a tremendous achievement Standard 81 – 91 that they should be proud of. Through excellent customer Hemostats 92 – 97 service, impeccable product quality and a passionate team, FST has become a household name in surgical and microsurgical instruments and accessories. During the COVID19 pandemic and the following worldwide lockdown, whether at their home office or on-site, our teams Probes & Hooks 99 – 103 around the world were able to provide our customers with the Spatulae 102 – 105 instruments and accessories they needed. Under challenging Hippocampal Tools & Spoons 106 – 107 circumstances, we kept our warehouses open in order to ship Pins & Holders 108 – 109 products to research laboratories, biotech’s and academic Wound Closure 110 – 121 institutions around the world while our support staff actively Needle Holders & Suture 110 – 118 Staplers, Clips & Applicators 119 – 121 continued to provide assistance to all customer questions and Retractors 122 – 129 inquiries.