ARIA® Spinal System Surgical Technique
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product Catalog Stainless Steel Vaginal Specula
PRODUCT CATALOG STAINLESS STEEL VAGINAL SPECULA Graves Speculum Product No. Description LTL-GS300 Graves Speculum, Small 3” x .75” LTL-GS400 Graves Speculum, Medium 4” x 1.5” LTL-GS450 Graves Speculum, Large 4.50” x 1.5” LTL-GS700 Graves Speculum, XL 7” x 1.5” Pederson Speculum Product No. Description LTL-PS305 Pederson Speculum, Virginal 3” x .5” LTL-PS300 Pederson Speculum, Small 3” x 1” LTL-PS400 Pederson Speculum, Medium 4” x 1” LTL-PS450 Pederson Speculum, Large 4.5” x 1” LTL-PS455 Pederson Speculum, Extra Narrow 4.5” x .5” LTL-PS700 Pederson Speculum, XL 7” x 1” Open Sided Speculum Product No. Description LTL-WGR400 Weisman-Graves Speculum, Medium, Right Open 4” x 1.5” LTL-WGR450 Weisman-Graves Speculum, Large, Right Open 4.5” x 1.5” LTL-WGL400 Weisman-Graves Speculum, Medium, Left Open 4” x 1.5” LTL-WGL450 Weisman-Graves Speculum, Large, Left Open 4.5” x 1.5” LTL-WPR400 Weisman-Pederson Speculum, Medium, Right Open 4” x 1” LTL-WPR450 Weisman-Pederson Speculum, Large, Right Open 4.5” x 1” LTL-WPL400 Weisman-Pederson Speculum, Medium, Left Open 4” x 1” LTL-WPL450 Weisman-Prderspm Speculum, Large, Left Open 4.5” x 1” *We also offer wide view (4cm) and full view (7cm) openings. 1 | TOLL FREE 1 [800] 910-8303 FAX 1 [805] 579-9415 WWW.LTLMEDICAL.NET BIOPSY PUNCHES Standard Style Rotating Style Tischler [Morgan] 7mm x 3mm Baby Tischler 4mm x 2mm Tischler Kevorkian 9.5mm x 3mm Product No. Description Product No. Description Product No. -

Inhaltsverzeichnis Index Index Indice Alfabético Indice
Inhaltsverzeichnis Index Index Indice alfabético Indice Inhaltsverzeichnis Index Index Indice alfabético Indice E-01 Inhaltsverzeichnis Index Index Indice alfabético Indice A B Accessories for sterilization container ......... 88-38 to 88-41 BABCOCK seizing forceps ........................................ 64-02 Adenotome LAFORCE .............................................. 46-19 BABINSKY percussion hammer ............................... 02-07 ADLERKREUTZ thumb and tissue forceps ............... 10-04 BACKHAUS-CLIP tube holder towel clamp .............. 14-03 ADSON BABY hemostatic forceps ............................ 12-09 BACKHAUS KOCHER towel clamp .......................... 14-02 ADSON-Baby retractor .............................................. 18-15 BACKHAUS towel clamp ........................................... 14-02 ADSON BAGGISH uterine biopsy specimen forceps ............. 70-45 bone rongeur ........................................................ 32-03 BAILEY-BABY rib contractor ..................................... 56-18 ADSON-BROWN thumb and tissue forceps ............. 10-03 BAILEY-GIBBON rib contractor ................................. 56-18 ADSON BAILEY rib contractor ............................................... 56-18 elevator ................................................................. 32-21 BAINBRIDGE hemostatic forceps ............................................... 12-09 atraumatic forceps ................................................ 13-09 hypophyseal forceps ............................................ -

AUA BLUS Handbook of Laparoscopic and Robotic Fundamentals
AUA BLUS Handbook of Laparoscopic and Robotic Fundamentals Sean Collins, Daniel S. Lehman, Elspeth M. McDougall, Ralph V. Clayman, and Jaime Landman ©American Urological Association Education & Research, Inc. Table of Contents 1. Introduction 2. Patient selection a. Indication b. contradindications c. special considerations 3. Physiologic effects of pneumoperitoneum a. Renal surgery transperitoneal b. Renal surgery retroperitoneal c. Hand-assisted laparoscopic nephrectomy d. Prostatectomy 4. Getting Started 5. Patient positioning a. Renal surgery transperitoneal b. Renal surgery retroperitoneal c. Hand-assisted laparoscopic nephrectomy d. Prostatectomy 6. Strategic placement of surgical team and operating room (OR) equipment 7. Access a. Primary access b. Renal surgery transperitoneal trocar placement c. Renal surgery retroperitoneal trocar placement d. Secondary access e. Retroperitoneal primary and secondary access f. Hand-assisted laparoscopic nephrectomy trocar placement g. Prostatectomy trocar placement 8. Instrumentation a. Trocars i. Cutting ii. Dilating iii. Radially dilating b. Bipolar cautery c. Monopolar cautery d. Ultrasonic instrumentation e. Vessel sealing devices i. LigaSure ii. Enseal f. Staplers g. Vascular clamps h. Suture anchors i. Titanium clips j. Locking clips q. Retractors r. Hemostatic agents s. Hand Assisted devices 2 9. Technique for Transperitoneal Laparoscopic Nephrectomy 10. Complications of laparoscopic surgery 3 Chapter 1. Introduction The American Urological Association (AUA) has prepared this handbook for all those new to laparoscopy. Rather than being a detailed surgical atlas, this is a handbook designed to introduce the fundamental principles of laparoscopy including: indications and contraindications for laparoscopy, the physiologic effects of pneumoperitoneum, patient positioning; abdominal access and trocar placement; strategic placement of the operating room (OR) team and equipment, overview of laparoscopic instrumentation, and complications unique to laparoscopic surgery. -

11 Cardiac Instruments
FEHLING AORTIC PUNCHES INS TRUMENTS AORTENSTANZEN 11/1 FEHLING... ... THE DIFFERENCE INSTRUMENTS FOR THORACIC, CARDIAC AND VASCULAR SURGERY INSTRUMENTE FÜR THORAX-, HERZ- UND GEFÄSSCHIRURGIE FEHLING Hanauer Landstraße 7A · 63791 Karlstein/Germany · www.fehling-instruments.de INSTRUMENTS +49(0) 6188 - 9574.40 +49(0) 6188 - 9574.45 [email protected] FEHLING STERNAL RETRACTORS INSTRUMENTS STERNUMSPREIZER 11/2 CALAFIORE STERNAL RETAINER STERNUMOFFENHALTER 1 1 ⁄2 ⁄4 1 1 1 ⁄16 ⁄2 ⁄2 STERNUM BLADE SCREW NUT STERNUMBLATT FLAT WRENCH STORAGE TRAY MUTTER GABELSCHLÜSSEL LAGERUNGS- LEFT RIGHT SINGLE USE BEHÄLTER LINKS RECHTS MPA-5 MPC-1L MPC-1R NEONATAL 7 x 30 mm** MPB-1 7 x 30 mm* 7 x 30 mm* Ø 8 MPC-0P MPB-7L MPB-7R 10 x 18 mm* 10 x 18 mm* MPA-6 “PEDIATRIC“ PEDIATRIC 45 x 65 mm** MPB-2 MPA-2L MPA-2R Ø 12 10 x 50 mm* 10 x 50 mm* MPA-3L MPA-3R ADULT 15 x 70 mm* 15 x 70 mm* MPA-9 45 x 65 mm** MPC-0A Ø 16 “ADULT“ MPA-4L MPA-4R ADIPOSIS 20 x 100 mm* 20 x 100 mm* MPA-7 70 x 90 mm** MPB-3 Ø 16 MPB-5L MPB-5R 15 x 30 mm* 15 x 30 mm* MPA-8 MPC-0C OSTEOPOROSIS 95 x 115 mm** “CURVED“ MPB-6L MPB-6R Ø 16 20 x 30 mm* 20 x 30 mm* *blade size / Blattgröße **opening width / Öffnungsweite exemplary configuration exemplary configuration Beispielkonfiguration Beispielkonfiguration ADULT - ADIPOSIS OSTEOPOROSIS FEHLING RETRACTORS INSTRUMENTS SPREIZER 11/3 TILTING KIPPBAR FOR PARTIAL STERNOTOMY FÜR PARTIELLE STERNOTOMIE BLADE SIZE BLATTGRÖSSE SPREADING WIDTH 160 mm a x b SPREIZWEITE 100 mm 35 x 50 mm MRM-5 45 x 50 mm MRM-6 215 mm 1 ⁄3 MARJAN 2 ⁄3 SUPERFLEX SOFT TISSUE RETRACTOR WEICHGEWEBERETRAKTOR 200 x 25 mm MTI-0 2 ⁄3 STERILISATION AND STORAGE TRAY FOR MTI-0 STERILISIER- UND LAGERSIEB FÜR MTI-0 220 x 51,2 x 19,5 mm MTK-0 FEHLING PROBES/SIZERS INSTRUMENTS SONDEN/LEHREN 11/6 SUPERPLAST A 45 mm A for mitral valve repair techniques without prosthetic ring SUPERPLAST instruments are construc- für Mitralklappenrekonstruktion ted of shape-memory metal. -

Fine Surgical Instruments for Research™
FINE SCIENCE TOOLS CATALOG 2021 FINE SCIENCE TOOLS CATALOG FINE SURGICAL INSTRUMENTS FOR RESEARCH™ TABLE OF CONTENTS | CATALOG 2021 Scissors 3 – 35 Spring 3 – 14 Fine 15 – 28 Letter from the Managing Partner Surgical 29 – 35 Bone Instruments 36 – 49 Rongeurs 36 – 38 Dear Customers, Cutters 39 – 47 Other Bone Instruments 47 – 48 After my uncle and founder of Fine Science Tools, Hans, handed Curettes & Chisels 49 over the management of the company to my cousin Rob and I Scalpels & Knives 50 – 61 last year, a lot has happened. Coming to FST as an “outsider”, my primary goal was to learn everything about the products, customers and the entire company. From my very first day, Forceps 63 – 91 I learned just how much my uncle Hans and the excellent Dumont 63 – 73 managerial team, Rob, Michael and Christina were able to Fine 72 – 80 grow the company over the last 45 years from a single office in Moria 74 S&T 75, 77 Vancouver into a global enterprise, a tremendous achievement Standard 81 – 91 that they should be proud of. Through excellent customer Hemostats 92 – 97 service, impeccable product quality and a passionate team, FST has become a household name in surgical and microsurgical instruments and accessories. During the COVID19 pandemic and the following worldwide lockdown, whether at their home office or on-site, our teams Probes & Hooks 99 – 103 around the world were able to provide our customers with the Spatulae 102 – 105 instruments and accessories they needed. Under challenging Hippocampal Tools & Spoons 106 – 107 circumstances, we kept our warehouses open in order to ship Pins & Holders 108 – 109 products to research laboratories, biotech’s and academic Wound Closure 110 – 121 institutions around the world while our support staff actively Needle Holders & Suture 110 – 118 Staplers, Clips & Applicators 119 – 121 continued to provide assistance to all customer questions and Retractors 122 – 129 inquiries. -

Outpatient Surgical Procedures – Site of Service: CPT/HCPCS Codes
UnitedHealthcare® Commercial Policy Appendix: Applicable Code List Outpatient Surgical Procedures – Site of Service: CPT/HCPCS Codes This list of codes applies to the Utilization Review Guideline titled Effective Date: August 1, 2021 Outpatient Surgical Procedures – Site of Service. Applicable Codes The following list(s) of procedure and/or diagnosis codes is provided for reference purposes only and may not be all inclusive. The listing of a code does not imply that the service described by the code is a covered or non-covered health service. Benefit coverage for health services is determined by the member specific benefit plan document and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claim payment. Other Policies and Guidelines may apply. This list contains CPT/HCPCS codes for the following: • Auditory System • Female Genital System • Musculoskeletal System • Cardiovascular System • Hemic and Lymphatic Systems • Nervous System • Digestive System • Integumentary System • Respiratory System • Eye/Ocular Adnexa System • Male Genital System • Urinary System CPT Code Description Auditory System 69100 Biopsy external ear 69110 Excision external ear; partial, simple repair 69140 Excision exostosis(es), external auditory canal 69145 Excision soft tissue lesion, external auditory canal 69205 Removal foreign body from external auditory canal; with general anesthesia 69222 Debridement, mastoidectomy cavity, complex (e.g., with anesthesia or more -

Surgical Technique Guide Description
Surgical Technique Guide Description The PrimaLIF™ LIFF Interbody Fusion System is a PEEK interbody cage system implanted to replace collapsed, damaged, or unstable vertebral discs due to degenerative conditions, tumor or trauma. The system is designed to provide anterior spinal column support and bone graft containment to promote bony fusion. This Lateral Lumbar Interbody Fusion (LLIF) system includes insertion instruments for the interbody implant, a retractor system, table arm, and disc preparation instruments. Indications for Use The OsteoMed Spine PrimaLIF™ LLIF is indicated for intervertebral body fusion of the lumbar spine to be used with autogenous bone graft, from L2 to S1, in skeletally mature patients who have had six months of non-operative treatment. The device is intended for use at either one level or two contiguous levels for the treatment of degenerative disc disease (DDD) and these patients may have up to Grade I spondylolisthesis or retrolisthesis at the involved levels. DDD is defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies. The device is intended for use with supplemental fixation such as PrimaLOK™ SP and PrimaLOK™ FF which has been cleared for use in the lumbar spine. For additional information, warnings and contraindications, please refer to the Product Insert. TABLE OF CONTENTS Product Labeling Description Inside Indications for Use Cover Design Rationale Rethinking Lateral Access 1 Retractor Highlights 1 Interbody Implant Highlights 2 Surgical -

Equine Catalog
Dear Customer, Sontec Instruments, Inc. is a family owned & operated medical company, providing personalized service featuring the finest in surgical instrumentation for over half a century. Our surgical instruments encompass the entire human anatomy including specialty products specific to small and large animal surgery. Owner of Sontec Instruments, Dennis Russell Scanlan III and his sons, Johann, Stefan and Angus Scanlan bring with them a lifetime of experience creating the highest quality products made by the world’s leading manufacturing facilities featuring, cutting edge robotic technology, handmade workmanship combined with an understanding how to make exactly what our valued customers have come to expect. Dennis R. Scanlan, III President & CEO and his wife Caron C. Scanlan thank you, for the opportunity to present our specialty Equine catalog. Sincerely, Dennis Russell Scanlan III Printed 8/20 Colorado, USA / 1.800.821.7496 / www.SontecInstruments.com 1 Table of Contents Important Information . 4 Rongeurs ..............33 Equine Specialty . 55 Forceps . 121 Retractors ............149 Scissors. 159 Needle Holders . 209 Index ................228 Colorado, USA / 1.800.821.7496 / www.SontecInstruments.com 3 IMPORTANT INFORMATION Troubleshooting Guide Guarantee & Repairs Policy System Needle Holders • Equine (Arthroscopic) • Repair is necessary when needle holder Problem Cause Solution Sontec® surgical instruments are guaran- • Eye no longer securely holds needle when teed to be free of defects in materials and • Neurology & Orthopedic locked on the second ratchet tooth, and workmanship. Any Sontec® instrument that • Orthopedic & Arthroscopic needle turns easily by hand Rust Worn chrome plating on Be aware of plating condition and remove from is defective will be repaired or replaced at our • Urology brass instruments service when wear is visible. -
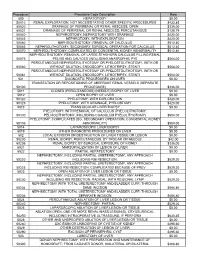
Procedure Procedure Code Description Rate 500
Procedure Procedure Code Description Rate 500 HEPATOTOMY $0.00 50010 RENAL EXPLORATION, NOT NECESSITATING OTHER SPECIFIC PROCEDURES $433.85 50020 DRAINAGE OF PERIRENAL OR RENAL ABSCESS; OPEN $336.00 50021 DRAINAGE OF PERIRENAL OR RENAL ABSCESS; PERCUTANIOUS $128.79 50040 NEPHROSTOMY, NEPHROTOMY WITH DRAINAGE $420.00 50045 NEPHROTOMY, WITH EXPLORATION $420.00 50060 NEPHROLITHOTOMY; REMOVAL OF CALCULUS $512.40 50065 NEPHROLITHOTOMY; SECONDARY SURGICAL OPERATION FOR CALCULUS $512.40 50070 NEPHROLITHOTOMY; COMPLICATED BY CONGENITAL KIDNEY ABNORMALITY $512.40 NEPHROLITHOTOMY; REMOVAL OF LARGE STAGHORN CALCULUS FILLING RENAL 50075 PELVIS AND CALYCES (INCLUDING ANATROPHIC PYE $504.00 PERCUTANEOUS NEPHROSTOLITHOTOMY OR PYELOSTOLITHOTOMY, WITH OR 50080 WITHOUT DILATION, ENDOSCOPY, LITHOTRIPSY, STENTI $504.00 PERCUTANEOUS NEPHROSTOLITHOTOMY OR PYELOSTOLITHOTOMY, WITH OR 50081 WITHOUT DILATION, ENDOSCOPY, LITHOTRIPSY, STENTI $504.00 501 DIAGNOSTIC PROCEDURES ON LIVER $0.00 TRANSECTION OR REPOSITIONING OF ABERRANT RENAL VESSELS (SEPARATE 50100 PROCEDURE) $336.00 5011 CLOSED (PERCUTANEOUS) (NEEDLE) BIOPSY OF LIVER $0.00 5012 OPEN BIOPSY OF LIVER $0.00 50120 PYELOTOMY; WITH EXPLORATION $420.00 50125 PYELOTOMY; WITH DRAINAGE, PYELOSTOMY $420.00 5013 TRANSJUGULAR LIVER BIOPSY $0.00 PYELOTOMY; WITH REMOVAL OF CALCULUS (PYELOLITHOTOMY, 50130 PELVIOLITHOTOMY, INCLUDING COAGULUM PYELOLITHOTOMY) $504.00 PYELOTOMY; COMPLICATED (EG, SECONDARY OPERATION, CONGENITAL KIDNEY 50135 ABNORMALITY) $504.00 5014 LAPAROSCOPIC LIVER BIOPSY $0.00 5019 OTHER DIAGNOSTIC PROCEDURES -

DILAPAN-S® Hygroscopic Cervical Dilator Instructions For
DILAPAN-S® Hygroscopic Cervical Dilator Re-use / re-sterilization / reprocessing1) of the DILAPAN-S® single-use medical device may result in physical damage to the medical device, failure of intended use of the medical device, and illness or injury to the patient as a result of infection, inflammation Instructions for Use and / or disease due to product contamination, infections and insufficient sterility of the product. GENERAL INFORMATION 1) A process carried out on a used device in order to allow its safe reuse including cleaning. Content Careful placement of the device is essential to avoid traumatic injury to the cervix or A sterile hygroscopic cervical dilator packed in a printed composite primary peel-open uterus and to avoid migration of the device either upward into the uterus or downward pouch, a piece of Instructions for use. into the vagina. ® The DILAPAN-S® is available in a box of 25 dilators and in the following dimensions: The DILAPAN-S may fragment during removal using incorrect technique. Fragmentation 4×65 mm, 4×55 mm, 3×55 mm. may result in pieces of the device being retained in the uterus. Carefully follow the Removal instructions. Device description and performance Do not use if primary packaging has been opened or damaged. Synthetic hydrogel cervical dilator consists of the dilating part, the polypropylene handle and the marker string (see the figure below). The dilating part is manufactured from Do not re-use, intended for one-time use. an anisotropic xerogel of AQUACRYL. The dilator is capable of increasing in diameter as it absorbs moisture from the genital tract. -

2019 Compilation of Inpatient Only Procedure Lists by Specialty
2019 Compilation of Inpatient Only Procedure Lists by Specialty (for CPT searching) 2019 Bariatric Surgery: Is the Surgery Medicare Inpatient Only or not? Disclaimer: This is not the CMS Inpatient Only Procedure List (Annual OPPS Addendum E). No guarantee can be made of the accuracy of this information which was compiled from public sources. CPT Codes are property of the AMA and are made available to the public only for non-commercial usage. Gastric Bypass or Partial Gastrectomy Procedures Inpatient Only Procedure Not an Inpatient Only Procedure 43644 Laparoscopy, surgical, gastric restrictive 43659 Unlisted laparoscopy procedure, stomach procedure; with gastric bypass and Roux-en-Y gastroenterostomy (roux limb 150 cm or less) 43645 Laparoscopy, surgical, gastric restrictive procedure; with gastric bypass and small intestine reconstruction to limit absorption 43775 Laparoscopy, surgical, gastric restrictive procedure; longitudinal gastrectomy (ie, sleeve gastrectomy) 43843 Gastric restrictive procedure, without gastric bypass, for morbid obesity; other than vertical- banded gastroplasty 43845 Gastric restrictive procedure with partial gastrectomy, pylorus-preserving duodenoileostomy and ileoileostomy (50 to 100 cm common channel) to limit absorption (biliopancreatic diversion with duodenal switch) 43846 Gastric restrictive procedure, with gastric bypass for morbid obesity; with short limb (150 cm or less) Roux-en-Y gastroenterostomy 43847 Gastric restrictive procedure, with gastric bypass for morbid obesity; with small intestine reconstruction -

Instruments 449-478 4/3/06 10:42 AM Page 449
Instruments_449-478 4/3/06 10:42 AM Page 449 Neuro Hammers & Diagnostic ADC® NEUROLOGICAL HAMMERS Four of the most popular hammers for diagnosis of neurological function. 369110105375 Buck Hammer, 7 1/4˝, Chrome Plated Handle w/2 sided rubber head, Handle Conceals “screw-in” Brush, Needle Contained Within The Head 369310105374 Taylor Hammer, 7 1/2˝, Chrome Handle w/triangular rubber head, Orange 3693BK10141795 Taylor Hammer, 7 1/2˝, Chrome Handle w/triangular rubber head, Black 3693DG10141796 Taylor Hammer, 7 1/2˝, Chrome Handle w/triangular rubber head, Dark Green 3693RB10141797 Taylor Hammer, 7 1/2˝, Chrome Handle w/triangular rubber head, ADC® TUNING FORKS Royal Blue 369510105372 Wartenberg Pinwheel, 7 1/2˝, Stainless Steel Handle w/textured grip, Non magnetic, corrosion resistant aluminum alloy construction weighs 1/3 of Rotating Spur comparable steel tuning forks. Produced from 3/8˝ x 1˝ bar stock for superior 369710105373 Babinski Hammer, 8 1/2˝, Octagonal Stainless Steel Handle w/concealed performance and consistent frequency accuracy. Extra long 2˝ handle of turned needle, Rubber Head smooth aluminum to facilitate bone conduction tests. 50012810105366 Tuning Fork w/fixed weight, 128cps Frequency 50025610105367 Tuning Fork w/fixed weight, 256cps Frequency 50051210105368 Tuning Fork w/o weight, 512cps Frequency 50102410105369 Tuning Fork w/o weight, 1024cps Frequency 50204810105370 Tuning Fork w/o weight, 2048cps Frequency 50409610105371 Tuning Fork w/o weight, 4096cps Frequency 1-200 1-220 MILTEX HAMMERS 1-20010090643 Taylor Percussion