A Morphological and Histometrical Study on Distribution and Heterogeneity of Mast Cells of Chicken’S and Quail’S Digestive Tract *
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Life Science Journal 2013;10(2) 479
Life Science Journal 2013;10(2) http://www.lifesciencesite.com Histological Observations on the Proventriculus and Duodenum of African Ostrich (Struthio Camelus) in Relation to Dietary Vitamin A. Fatimah A. Alhomaid1 and Hoda A. Ali2 1Dept. of Biology, Collage of Science and Arts, Qassim University, KSA 2Dept. of Nutrition and Food Science, Collage of Designs and Home Economy, Qassim University, KSA. Email: [email protected]; [email protected] Abstract: Research problem: The fine structure of the gut in different avian species in relation to dietary vitamins status have widely been studied with the exception of ostriches. Objectives: To study the histological structure of the African ostrich proventriculus and duodenum in relation to two levels of vitamin A (7500 and 9000) IU/kg diet using light microscope. Methods: Twenty male African ostriches with average age 65-67 weeks and apparently healthy were used. They were divided into two equal groups, the first one received diet adjusted to supply 7500 IU/kg diet vitamin A. The second group fed diet formulated to furnished 9000 IU/Kg diet vitamin A. Both diets were isonitrogenous and isocaloric. Body weight gain, feed intake and feed conversion rate were calculated. At the end of four weeks, pieces from the different parts of proventriculus and duodenum were taken for light microscopic examinations. Result: Body weight gain and feed conversion rate were improved in group received 9000IU of vitamin A comparable to group fed 7500IU. Histological structure of proventriculus of birds receiving 7500 IU/Kg vitamin A showing vascular congestion, thinning connective tissue and sloughing of the columnar epithelia lining the central collecting duct. -

Histogenesis of the Proventricular Submucosal Gland of the Chick As Revealed by Light and Electron Microscopy Dale S
HISTOGENESIS OF THE PROVENTRICULAR SUBMUCOSAL GLAND OF THE CHICK AS REVEALED BY LIGHT AND ELECTRON MICROSCOPY DALE S. THOMSON Malone College, Canton, Ohio ABSTRACT The development of the submucosal tubuloalveolar glands of the proventriculus was studied with both light and electron microscopes, with special reference given to the 13-18-day period. Beginning as an invagination in the stratified columnar epithelium lining the lumen of the proventriculus, the simple tubular gland, by repeated bifurcation of the invaginated portion, by sloughing of the superficial cells, and by remodeling of the residual basal cells from columnar to cuboidal, becomes a compound tubuloalveolar gland composed of numerous secretory units. Concomitant with the gross cellular changes, ultra- structural changes in the intercellular membranes are evident. By day 4 after hatching, the cells forming the secretory units appear to be functional. INTRODUCTION The avian stomach is peculiar in that it consists of two morphologically and physiologically distinct parts: the glandular portion, or proventriculus, and the muscular portion, or gizzard. The proventriculus is a relatively thick-walled structure located at the distal end of the oesophagus and containing both simple mucous-secreting glands and compound submucosal tubuloalveolar glands. The gizzard is a thick muscular bulb, the mucosa of which contains simple glandular cells which secrete a tough, horny layer. The proventriculus has been described by Cazin (1886, 1887), Zietschmann (1908), Calhoun (1933, 1954), Batt (1924), and Bradley and Graham (1950). Aitken (1958) and Toner (1963) further amplified the literature with specific reference to the compound submucosal glands. Sjogren (1941) and Hibbard (1942) described the embryonic development of the submucosal glands. -

Gastrointestinal Tract Morphometrics and Content of Commercial and Indigenous Chicken Breeds with Differing Ranging Profiles
animals Article Gastrointestinal Tract Morphometrics and Content of Commercial and Indigenous Chicken Breeds with Differing Ranging Profiles Joanna Marchewka 1,*, Patryk Sztandarski 1 , Zaneta˙ Zdanowska-S ˛asiadek 1, Dobrochna Adamek-Urba ´nska 2 , Krzysztof Damaziak 3, Franciszek Wojciechowski 1, Anja B. Riber 4 and Stefan Gunnarsson 5 1 Institute of Genetics and Animal Biotechnology, Polish Academy of Sciences, Jastrz˛ebiec, 05-552 Magdalenka, Poland; [email protected] (P.S.); [email protected] (Z.Z.-S.);˙ [email protected] (F.W.) 2 Department of Ichthyology and Biotechnology in Aquaculture, Institute of Animal Science, Warsaw University of Life Sciences, 02-786 Warsaw, Poland; [email protected] 3 Department of Animal Breeding, Faculty of Animal Breeding, Bioengineering and Conservation, Institute of Animal Science, Warsaw University of Life Sciences, 02-786 Warsaw, Poland; [email protected] 4 Department of Animal Science, Aarhus University, DK-8830 Aarhus, Tjele, Denmark; [email protected] 5 Department of Animal Environment and Health, Swedish University of Agricultural Sciences (SLU), S-532 23 Skara, Sweden; [email protected] * Correspondence: [email protected] Simple Summary: Differences in the range use by poultry exist on the individual or breed level, even if equal opportunity of outdoor access is provided. Birds reared with access to the pasture consume some of Citation: Marchewka, J.; Sztandarski, the material found outdoors, such as plants, insects, and stones. The frequency of outdoor range use may P.; Zdanowska-S ˛asiadek, Z.;˙ Adamek-Urba´nska,D.; Damaziak, K.; be associated with the ingested material and the development of the bird gut. -

Avian Digestion
4/5/2012 Avian digestion I. Introduction A. Shapes II. Taxonomy of food habits B. Grinding Function A. Herbivores C. Crop Milk B. Carnivores D. Other Functions A wonderful bird is the pelican, III. Mouth and Pharynx V. Stomach His bill will hold more than his belly can. A. Bill A. Proventriculus He can take in his beak B. Palate B. Ventriculus Food enough for a week, C. Tongue VI. Small Intestine But I'm damned if I see how the hell he D. Salivary Glands A. Duodenum can. IV. Esophagus and Crop B. Function A. Storage --Dixon Lanier Merritt (1879-?) The Pelican (1910) I. Introduction Digestive system needs to be as light as possible High metabolic rates require large extremely efficient amounts of fuel warbler might eat 80 percent of its body weight in a day! 1 4/5/2012 Loss of teeth and minimum jaw musculature Problem for birds – need to keep low body weight Thus, little fat storage need to locate, ingest, digest food as quickly and efficiently as possible Stellar’s Sea Eagle Major components of avian digestive system II. Taxonomy of food habits • oral cavity Many birds are generalists but many are also specialists • pharynx • esophagus (+ crop) Specializations are evident through the entire alimentary • stomach (proventriculus, canal. ventriculus) • small intestine • large intestine • cloaca 2 4/5/2012 As a group birds consume about any kind of food A. Herbivores ants nectar Granivores buds pollen Frugivores crustaceans roots Nectivores fish sap Folivores fruit snails grass wax insects etc. leaves B. Carnivores III. Mouth and Pharynx Raptors A. -

An Emerging Exotic Disease of Parrots in Australia AVIAN, UNUSUAL & EXOTIC
avj_109.fm Page 119 Thursday, February 15, 2007 9:35 AM AVIAN, UNUSUAL & EXOTIC Blackwell Publishing Asia CASE REPORT CORE Metadata, citation and similar papers at core.ac.uk Provided by University of QueenslandProventricular eSpace dilatation disease: an emerging exotic disease of parrots in Australia AVIAN, UNUSUAL & EXOTIC RJT DONELEY,a RI MILLERb and TE FANNINGa syndrome of weight loss associated with regurgitation and the Proventricular dilatation disease is a viral disease seen as a passage of undigested food in the faeces, other clinical signs also segmental neuropathy in parrots. It has always been believed include ataxia, abnormal head movements, progressive paresis, to be a disease exotic to Australia, with the only reported case proprioceptive deficits, anorexia, lethargy and, occasionally, being a legally imported Green Wing Macaw (Ara chloroptera) 3 in 1993. This paper reports a cluster of cases seen in south- sudden death. K Rosenthal (personal communication) and D east Queensland in 2005 to 2006. Clinical signs, autopsy Monks (personal communication) consider that, in contrast to findings and histopathological findings are described. No other species, polyuria and polydipsia are frequently seen in Gray pattern or common source for these cases could be identified. parrots (Psittacus erithacus erithacus) affected with PDD. The implications for Australian aviculture and avifauna are discussed. A variety of aetiological agents have been proposed, including paramyxovirus, togavirus, adenovirus, coronavirus,4 and Eastern Key words: proventricular dilatation disease, psittacines, parrots, exotic disease Equine Encephalitis virus. Other suggested aetiologies included Aust Vet J 2007;85:119–123 doi: 10.1111/j.1751-0813.2007.00109.x immune-mediated reactions to an unknown viral agent, how- ever, no consistent viral isolation or serological findings have AST Aspartate aminotransferase confirmed the involvement of any one virus. -

The Internal Anatomy of the Silverfish Otenclepisma Campbelli Barnhart and Lepisma Saccharina Linnaeus (Thysanura: Lepismatidae)
THE INTERNAL ANATOMY OF THE SILVERFISH OTENCLEPISMA CAMPBELLI BARNHART AND LEPISMA SACCHARINA LINNAEUS (THYSANURA: LEPISMATIDAE) DISSERTATION Presented in Pertial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By CLYDE STERLING BARNHART, SR., B.Sc., M.Sc The Ohio State University 1958 Approved byj Department PREFACE In 19^7 the writer began a study of the trachea- tion of a silverfish collected from the Main Library on the Ohio State University campus. This began under the direction of the late Dr. C. H. Kennedy, professor of Entomology, the Ohio State University, in his course on Insect anatomy. Professor Kennedy was Impressed with the minute detail with which the tracheation could be traced since this insect was so small. It was his interest and encouragement which prompted the writer to continue this work beyond the course and later to expand it into the more complete study embodied in this dissertation. The writer is grateful to the late professor Kennedy for his part in providing the original encouragement for this study. The writer wishes also to express his sincere gratitude to Dr. Donald J. Borror, professor of Entomo logy* The Ohio State University, for his helpful guidance and suggestions in bringing the work of this dissertation to completion. li TABLE CP CONTENTS Pege INTRODUCTION................................... 1 MATERIALS AND METHODS........................... 2 TIES RESPIRATORY SYSTEM.......................... 4 THE ALIMENTARY CANAL............................ 14 THE CENTRAL NERVOUS SYSTEM...................... 24 THE DORSAL VESSEL............................... 28 THE REPRODUCTIVE ORGANS......................... 32 ABBREVIATIONS USED ON FIGURES.................... 40 FIGURES........................................ 43 SUMMARY......................................... 65 BIBLIOGRAPHY.................................... 68 ill LIST OF ILLUSTRATIONS Figure Page 1 Tracheation of the head, thorax, and first abdominal segment of C . -

Comparative Microscopic Study of the Proventriculus and Duodenum Of
CO^!PARATIVI? MICROSC0i»IC STLOT CF THE PROVl'HTfilCULlJS AHi> Db'ODFKUM OF THE MOUHNXm DOVE, RIUMHKADT^D ^iOODFKCK^ ASXi U&ADOKIMX &• S«* Bethany College, 1940 A TRESIS ubmitted In pertlal rvilfiUment of the requiresiente for the degree <^ mSTEIt OF SCIEHGK Depertiaent of zoology KAKSAS STATE COLI^BOK AORICULTURK AUD APi» 1,113) 3CIEKCB 1946 c. d ZKmODtJCTXOB X IIA7EHIALS m MBf!«3D8« •••••••• 1 DISCUSS XQH m) FRESEI^ATXOK <F PATA* 3 Bictology of Proventricultui ••••••••• 3 HiatoXog;^ of PuodMium 10 Diets of Birds studied 14 Tabulated Information end Statletioal Treatment of Date •••••••••••••16 Itothod of Statistical Analysis* • • • • • 15 Discussion of Statistical Interpretation* 16 SUmURY • 33 AQimO^il^'.VQmm •••••••••• 35 LXTBRATIJBE CITED ••••••••••••••••96 PLATES 38 1 IHfRODUCTIOK Hi jHaklng the study of the proventrlculus and duodenum of the isoumlng dove^ red«*he&dod woodpecker^ and imeadovlark,! tlxe ap* IMPCMioh was a ocniE^ratitre aleroeoopio study in an attea^t to cor* relate data obtained with the feeding habits* fhese three epeolee Here ohoeen because of their eoeiparative similarity in siase and because of the known diversity of the feeding habits* the literature pertaining to the hletology of the digestive tract of birds le widely scattered and much is contributed by foreign authop8« Standard texts m sianeaalian histology are ratlier difficult to uee i^ien naklng application to the histolo^^ of the digestive tracts of birds because of tiie differences in general structure and cell structure* The laasnal has nottxing comparable -
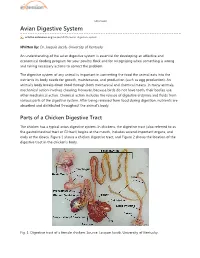
Avian Digestive System
eXtension Avian Digestive System articles.extension.org/pages/65376/avian-digestive-system Written by: Dr. Jacquie Jacob, University of Kentucky An understanding of the avian digestive system is essential for developing an effective and economical feeding program for your poultry flock and for recognizing when something is wrong and taking necessary actions to correct the problem. The digestive system of any animal is important in converting the food the animal eats into the nutrients its body needs for growth, maintenance, and production (such as egg production). An animal's body breaks down food through both mechanical and chemical means. In many animals, mechanical action involves chewing; however, because birds do not have teeth, their bodies use other mechanical action. Chemical action includes the release of digestive enzymes and fluids from various parts of the digestive system. After being released from food during digestion, nutrients are absorbed and distributed throughout the animal's body. Parts of a Chicken Digestive Tract The chicken has a typical avian digestive system. In chickens, the digestive tract (also referred to as the gastrointestinal tract or GI tract) begins at the mouth, includes several important organs, and ends at the cloaca. Figure 1 shows a chicken digestive tract, and Figure 2 shows the location of the digestive tract in the chicken's body. Fig. 1. Digestive tract of a female chicken. Source: Jacquie Jacob, University of Kentucky. Fig. 2. Location of the digestive tract in a female chicken. Source: Public domain. Beak/Mouth As with most birds, a chicken obtains feed by using its beak. Food picked up by the beak enters the mouth. -

Avian Gastrointestinal Anatomy and Physiology Kirk C
Avian Gastrointestinal Anatomy and Physiology Kirk C. Klasing, BS, MS, PhD A bird's gastrointestinal (GI) tract morphology, diges- their GI tracts appear to be morphologically tive strategy, and metabolic capability have been inti- more similar to chickens and turkeys than to mately intertwined during evolution to match the nutri- ent content and physical attributes of foods available in birds with very different dietary patterns, such as its natural habitat. The most commonly kept compan- carnivorous or herbivorous species. This permits ion species are granivorous with a tendency toward us to use the well-studied chicken as a point of omnivory. The beak, oral cavity, and tongue of granivo- reference when examining the GI tracts of com- rous birds have anatomic adaptations for shelling seeds, panion avian species. including ridges in the tomia of their beak for slicing the hull and a dexterous tongue for manipulating the seed The GI tract of a bird provides an environ- and disposing the hull. Granivorous species possess a ment for the physical and chemical reduction in sizeable crop or an expandable esophageal pouch for the size and molecular complexity of food and storing food so that large meals can be consumed. Their then absorbs the end products of digestion, proventriculus is somewhat small, and their gizzard is which are needed in widely differing quantities. large, muscular, and possesses a thick cuticle relative to carnivores or frugivores. A vestigial ceca and short A young chick requires a diet with more than rectum provide little area for use of symbiotic bacteria 15% protein, but only 0.0000003% of vitamin to aid in the digestion of the fibrous components of B12. -

A Comparison of Bird Digestive Systems by Diet
A Comparison of Bird Digestive Systems by Diet Research Thesis Presented in partial fulfillment of the requirements for graduation with research distinction in Biology in the undergraduate colleges of The Ohio State University by Adnan Siddique The Ohio State University November 2017 Project Advisor: Dr. Jacqueline K. Augustine, Department of Evolution, Ecology, and Organismal Biology Abstract The vertebrate digestive system can be modified to accommodate a diversity of diets. In general, herbivores tend to have longer, more complex digestive systems while carnivores have smaller, less elaborate digestive systems, but most of the research in this area examines mammalian morphology. Accordingly, the goal of this study was to determine the influence of diet on digestive system morphology in birds. I expected the size of the intestines, cecum (if present), proventriculus, and gizzard to be larger in herbivorous birds and smaller in carnivorous birds. Birds of varying diets were dissected and their digestive systems (intestines, cecum, proventriculus, and gizzard) were weighed. Additionally, each organ’s percent contribution to total body weight was calculated. Diet was determined by literature and by the gizzard contents of each bird. A mixed model was used for comparisons with the percentage of body mass due to organ weight as the dependent variable, bird diet as a fixed effect, and family as a random effect. Results from 34 birds revealed that diet affected size of the proventriculus but not the size of the intestines, gizzard, cecum, or the total digestive system. The proventriculus size was largest in insectivores and smallest in herbivores, with omnivores having an intermediately-sized proventriculus. -

The Digestive System of the Carolina Locust (Dissosteira Carolina Linn.) Harrison Morton Tietz University of Massachusetts Amherst
University of Massachusetts Amherst ScholarWorks@UMass Amherst Masters Theses 1911 - February 2014 1922 The digestive system of the Carolina locust (Dissosteira carolina Linn.) Harrison Morton Tietz University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/theses Tietz, Harrison Morton, "The digestive system of the Carolina locust (Dissosteira carolina Linn.)" (1922). Masters Theses 1911 - February 2014. 2033. Retrieved from https://scholarworks.umass.edu/theses/2033 This thesis is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Masters Theses 1911 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. •1 s I DATE DUE UNIVERSITY OF MASSACHUSETTS LIBRARY MORE ID -3234 HZ68 1923' T564 . TEE DIGESTIVE SYSTEM OF THE C^ROLUJA LOCUST (Diesosteira Carolina Linn.) HARRISOH M. TIETZ B. Sc. Submitted -^a a Thesis for the Degree of Master of Science MASSACHUSETTS AGRICULTURAL COLLIDE AMHERST , MASS 1922 ) THB AH TOUT OP MS DIGESTIVE SYSTEM 0? THE CAROLINA LOCUST (Dissosteira oarolina, Linn. By Harrison M.TIetz. Introduction. The typioal inaoot dissected by most students in biolopy Is the Carolina looust or some other common grasshopper. Many laboratory manuals describe the anatomy of these orthop- terons in more or leas detail but with tto few illustrations. This laok of illustrntions makes it diffioult for the instructor to present the subject and leads to confusion in the minds of the tudents. It is the aim of this paper to meet this need in part by presenting in its text and figures the results of a detailed study of the alimentary tract of the Carolina looust. -

Histochemical Structure of Stomach (Proventriculus and Gizzard) in Some Bird Species
Süleyman Demirel Üniversitesi Fen Bilimleri Enstitüsü Dergisi Suleyman Demirel University (2), 115-122, 2015 Journal of Natural and Applied Science 19 Histochemical Structure of Stomach (Proventriculus and Gizzard) in Some Bird Species Emel DEMİRBAĞ1*, Kenan ÇINAR1, Mehmet Ali TABUR1, Reşat Nuri AŞTI2 1Süleyman Demirel University, Faculty of Arts&Sciences, Department of Biology, Isparta 2Ankara University, Faculty of Veterinary, Department of Histology&Embryology, Ankara .03.2015 .04.2015) (Alınış Tarihi: 17 , Kabul Tarihi: 10 Keywords Abstract: (Accipiter nisus), crow (Corvus corone Passer domesticus) were Stomach samples (proventriculus and gizzard) of sparrow hawk CrowStomach ) and sparrow ( reaction to Sparrow hawk investigated in this study. In histochemical investigations, strong sulfated mucins Mucosubstance were not determined in compound glands of proventriculus, but a little AB (pH 0.5) in compound glands was observed in proventriculus of sparrow hawk observand crow. While weak AB (pH 1.0) (+) character was found in sparrow gizzard, it Histochemistry was found as dense in superficial glands of sparrow hawk. However, it was not ed in crow gizzard. While AF (+) character (sulfated mucins) was detected as dense in epithelium and as- weak in glands of gizzard of sparrow hawk, this character was not observed in glands of gizzards of crow and sparrow. Any reactivity to Periodic Acid Shiff (PAS) staining was not observed in both epithelium and glands of sparrow proventriculus. Bazı Kuş Türlerinde Mide (Proventrikulus ve Gizzard)’nin Histokimyasal Yapısı Anahtar Kelimeler: Özet: Accipiter nisus), karga (Corvus corone (Passer domesticus Atmaca Bu çalışmada atmaca ( ) ve serçeden KargaMide ) alınan mide örnekleri (proventrikulus ve gizzard) incelendi. Serçe Histokimyasal incelemelerde, proventrikulusun bileşik bezlerinde güçlü sülfatlı Mukosubstans musinlere rastlanmadı; fakat atmaca ve karga proventrikulusunda bileşik bezlerde Histokimya AB (pH 0.5)’e karşı zayıf bir reaksiyon gözlendi.