St Helena Bay Water Quality Trust
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phase 1 Archaeological Impact Assessment Proposed Development of Erf 36 St Helena Bay Saldanha -Vredenburg District1
PHASE 1 ARCHAEOLOGICAL IMPACT ASSESSMENT PROPOSED DEVELOPMENT OF ERF 36 ST HELENA BAY SALDANHA -VREDENBURG DISTRICT1 Prepared for WITHERS ENVIRONMENTAL CONSULTANTS Att: Mr Aubrey Withers PO Box 6118 Uniedal 7612 [email protected] .za Client: Northern Spark Trading (Pty) ltd By Jonathan Kaplan Agency for Cultural Resource Management P.O. Box 159 Riebeek West 7306 Ph/Fax: 022461 2755 DEPARTMENT OF CU Cellular : 082 321 0172 LTURAL AFFAlR\ &s p~ail : [email protected] RECEIVED/ONTVA . .""NG 26JUN 2009 PrIVat e 80 . P • Cope To;:/ KvOatsok X906 7 .f o oOpsla d 8t')O O EPT. KULTUURSAkE EN \ PO RT 1 MAY 2009 I Revised report Executive summary Withers Environmental Consultants requested that the Agency for Cultural Resource Management conduct a Phase 1 Archaeological Impact Assessment for a proposed retirement village type development on Erf 36 in St. Helena Bay. The subject property is currently zoned Fishing Industry and will be rezoned to General Residential to accommodate the proposed development. The property is vacant, but the demolished remains of a fishing factory are still visible on the proposed site. Much of the southern and eastern portion of the property is severely degraded, but the northern and western portion is still relatively undisturbed The following findings were made: A shell midden on top of a small dune hummock was documented directly alongside the fence line in the northern portion of the site. Shellfish occurs in open patches on the dune top and is also scattered fairly widely over the surrounding area. Stone tools in quartz, silcrete, quartzite and indurated shale were counted, but these are spread very thinly and unevenly over the area. -

What Lies Beneath Table Mountain Or All Models Are Wrong, but Some Are Useful
WHAT LIES BENEATH TABLE MOUNTAIN OR ALL MODELS ARE WRONG, BUT SOME ARE USEFUL Prof Alexander Kisters August 2016 WHAT LIES BENEATH TABLE MOUNTAIN OR ALL MODELS ARE WRONG, BUT SOME ARE USEFUL Inaugural lecture delivered on 2 August 2016 Prof Alexander Kisters Department of Earth Sciences Faculty of Science Stellenbosch University Editor: SU Language Centre Printing: SUN MeDIA ISBN: 978-0-7972-1614-3 Copyright © 2016 Alexander Kisters BIOGRAPHY rofessor Alexander (Alex) Kisters completed his Pgeology undergraduate studies and MSc at the Aachen University of Technology (RWTH) in Germany and obtained a PhD in geology from the University of the Witwatersrand, Johannesburg. Subsequently, he had worked in the mineral exploration industry in South Africa and had held various postdoctoral and lecturer positions at the universities of Aachen, Cologne and the Witwatersrand before joining Stellenbosch University in 1999. Currently, he is appointed at the Department of Earth Sciences, University of Stellenbosch, as a professor in structural geology and tectonics where he teaches under- and postgraduate courses. He has supervised and cosupervised over 50 honours students, 15 MSc studies and 7 PhD studies. Alex has held a National Research Foundation rating for the past 15 years and a B-rating for the last five years. His research focuses on a range of topics related to crustal deformation aimed at integrating field data with regional tectonic models and more generic processes of melt transport and hydrothermal fluid flow in the Earth’s crust. 1 ABSTRACT he geological evolution of the Western Cape remains elusive after over 100 years of research. Based on regional Tfieldwork and correlations, this contribution aims to develop a tectonic model for the deposition and deformation of rocks of the Malmesbury Group, the rocks that underlie much of the Western Cape. -
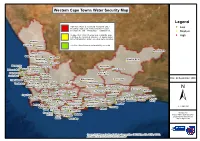
Legend High Risk: Water Demand and Availability Under (! Threat That Requires Urgent Interventions in Resource Low Development and Infrastructure Establishment
Western Cape Towns Water Security Map Legend High Risk: Water Demand and Availability under (! threat that requires urgent interventions in resource Low development and infrastructure establishment. (! Medium Medium Risk: Water Demand and Availability under (! High restriction due to lack of assurance of supply and/or lack of infrastructure and/or exceeding lawful allocation. Lutzville (! Vredendal (! Klawer Low Risk: Water Demand and Availability not at risk (! Murraysburg (! Lamberts Bay (! Graafwater (! (! Wuppertal Clanwilliam (! Beaufort West (! Redelinghuys Citrusdal (! (! Stompneus Eendekuil Merweville Bay Dwarskersbos (! ! (! (! (! Leeu-Gamka Britannia Bay ((!(!Velddrift (! (! Aurora (! Louwville Prince Paternoster (! (! Albert Road (! ! Piketberg Jacobsbaai (! ( (! (! (! (! Hopefield Porterville Saldanha ! Date: 04 September 2020 (! ( Matjies(!fontein Prince Albert Churchhaven Moorreesburg Gouda (! (! ! (! Tulbagh Touwsrivier ( (! Laingsburg Yzerfontein (! R(!iebeek-Wes (!(!Hermon (! (! De Doorns Zoar Darling (! Ceres (! Calitzdorp (! Riebeek-Kasteel Ladismith (! (! ! Dysselsdorp ( (! Oudtshoorn (! Uniondale Paarl (! Worcester Van Wyksdorp (! (! Haarlem (! Montagu (! Volmoed (! Robertson (! (! Cape Town Franschhoek (! Barrydale Karatara (! Ashton (! Ruitersbos ! Plettenberg (! (! (! (! ( Wittedrif Stellenbosch Gena(!dendal Greyton (! Groot Br(!akrivier !Bay(! (!(!(! Suurb(!ra(!ak Heidelberg Riversdale (! (! (! (! (! ( Natures Villiersdorp (! (! B(!randwag Wilderness Knysn(!a (! Bereaville(! (! Albertinia (! Valley ± Grabouw Riviersonderend Slangrivier (! (! (! (! (! Caledon Dana Bay Betty's Bay (! (! (! (! ! Botrivier Klipdale Witsand ( (! (! (! Vlees Bay 1 : 3 000 000 Hawston (! V(!ermo(!nt Napier Malgas Onrus (! Stilbaai He(!rmanus Elim (! Bredasdorp (!(! (! Franskraal Ga(!ns Bay (! Data Source: Arniston Dept of Water and Sanitation Strand Pearly L'Agulhas (!(! Dept of Local Government Beach Struis Bay Dept of Agriculture Source: Esri, Maxar, GeoEye, Earthstar Geographics, CNES/Airbus DS, USDA, USGS, AeroGRID, IGN, and the GIS User Community. -

Evidence for an Increasing Incidence and Severity of Harmful Algal Blooms in the Southern Benguela Region
Research Articles South African Journal of Science 103, May/June 2007 223 Evidence for an increasing incidence and severity of Harmful Algal Blooms in the southern Benguela region Vanessa C. Stephen and Philip A.R. Hockey winds,10,11 and is precisely the reason why HABs occur in this region so frequently. Harmful Algal Blooms (HABs) may lead to catastrophic mortality During blooms, extreme phytoplankton concentrations may over a range of trophic levels and impact on fisheries, local species’ cause mortalities across several trophic levels, regardless of populations, conservation management and the health of both whether the plankton themselves are toxic. Toxic blooms, gener- livestock and humans. Consequently, any increase in frequency ally comprising dinoflagellates or diatoms,7 impact either when and/or toxicity of these events is of concern. Recently this concern the phytoplankton are filtered as food by shellfish, resulting in has been realized, with reported increases in the frequency of HABs accumulation of toxins and their subsequent transfer up the from all continents except Antarctica. This reported rise is supported food chain (e.g. by Alexandrium catenella12), or when toxins are by data from the Benguela coast of western South Africa, where, released directly into the water (e.g. by Karenia cristata13). During since 1930, there has been a significant increase in the frequency of non-toxic blooms, mortality results either from eutrophication HABs and a slight increase in their average severity. There has been and subsequent anoxia, or from mechanical damage to gills. a sixfold increase in the number of HABs per decade since the 1960s, Decomposition of high densities of phytoplankton increases with the period 1990–2005 experiencing the greatest number of bacterial activity and reduces water oxygen content. -
Proposed Ad Hoc Amendment of Bergrivier Spatial Development Framework: Status Quo, 2012 - 2017
PROPOSED AD HOC AMENDMENT OF BERGRIVIER SPATIAL DEVELOPMENT FRAMEWORK: STATUS QUO, 2012 - 2017 COMPILED BY: CK RUMBOLL & PARTNERS JANUARY 2018 OUR REF: VEL/10146/AC Contents 1. Purpose and approach .......................................................................................................................... 1 2. Detailed Status Quo Analysis and Implications .................................................................................... 3 2.1 Biophysical Environment ............................................................................................................... 3 2.2 Socio- Economic Environment .................................................................................................... 10 2.3 Built Environment ........................................................................................................................ 19 3. Strengths, Weaknesses, Opportunities and Threats (SWOT) ........................................................... 35 4. Recommendation ................................................................................................................................. 38 5. Maps illustrating Status Quo Analysis ................................................................................................ 39 List of Graphs Graph 1: Sectoral GDPR contribution (% share) to West Coast Economy (Quantec 2015 - MERO, 2017) ..................................................................................................................................................................... -

Saldanha Bay Municipality V Britannia Beach Estate Pty
IN THE SUPREME COURT OF APPEAL OF SOUTH AFRICA JUDGMENT CASE NO: 796/11 Reportable In the matter between: SALDANHA BAY MUNICIPALITY APPELLANT and BRITANNIA BEACH ESTATE (PTY) LTD FIRST RESPONDENT BRITANNIA BAY DEVELOPERS (PTY) LTD SECOND RESPONDENT SANDY POINT BEACH PROPERTIES (PTY) LTD THIRD RESPONDENT WEST COAST MIRACLES (PTY) LTD FOURTH RESPONDENT Neutral Citation: Saldanha Bay Municipality v Britannia Beach Estate (Pty) Ltd (796/11) [2012] ZASCA 206 (30 November 2012) Coram: CLOETE and TSHIQI JJA and ERASMUS, SWAIN and MBHA AJJA Heard: 20 November 2012 Delivered: 30 November 2012 2 Summary: Municipal law – local government – the enforceability of conditions in respect of ‘capital contributions’ imposed in terms of s 42 of the Land Use Planning Ordinance 15 of 1985 and the various tariffs underlying such conditions. 3 ______________________________________________________________ ORDER ______________________________________________________________ On appeal from : Western Cape High Court, Cape Town (Cloete AJ sitting as court of first instance): 1 The appeal is upheld with costs, including the costs of two counsel. 2 The order of the court a quo is set aside and substituted with the following: ‘The application is dismissed with costs.’ ______________________________________________________________ JUDGMENT ______________________________________________________________ ERASMUS AJA (CLOETE and TSHIQI JJA and SWAIN and MBHA AJJA concurring): [1] This appeal arises from an order of the Western Cape High Court, Cape Town, declaring the tariff for the calculation of bulk infrastructure development contribution levies, set out in resolutions of the appellant’s council, to be of no force and effect; ordering the appellant to account to the respondents in respect of moneys levied by the appellant and paid by the respondents as contribution levies calculated in accordance with the impugned tariff; and ordering the appellant to pay the respondents’ costs. -

Sand Transport Along the Western Cape Coast
View metadata, citation and similar papers at core.ac.uk brought to you by CORE Diazville Beach), and terrigenous-rich Sand transport along the Western beaches north ofprovided Shelley by Cape Point Town (upUniversity to OpenUCT 96 wt% terrigenous material in St Helena Cape coast: gone with the wind? Bay). The decrease in terrigenous material between Cape Town and Saldanha Bay a,b* a reflects the reduced delivery of terrigenous Giuliana Franceschini , John S. Compton and sand by rivers. False Bay and Table Bay a Rochelle A. Wigley have perennial rivers that drain catch- ment areas receiving an average annual rainfall of 600 mm. Ephemeral rivers with EACH SAND SAMPLES WERE COLLECTED Sixteen Mile Beach), carbonate-rich small catchment areas drain the semi-arid Bbetween Cape Town and St Helena Bay beaches between Saldanha Bay and west coast region south of the Berg River in order to study sediment composition Shelley Point (up to 83 wt% CaCO at (annual rainfall is 273 mm at Langebaan). and transport along the Western Cape coast- 3 line. Between Cape Town and Saldhana Bay, the beaches are a mixture of terrigenous and carbonate material. Those between Saldanha Bay and Shelley Point are carbonate-rich. North of Shelley Point, terrigenous-rich beaches were found. The decrease in terri- genous material from Cape Townto Saldanha Bay is a reflection of reduced delivery of terrigenous sand by rivers. The low content of terrigenous material in beach sands between Saldanha Bay and Shelley Point is related to the high biogenic CaCO3 in the rocky coastal area. In St Helena Bay the high percentage of terrigenous material is contributed by the Berg River. -

Towards Integrated Coastal Management for Saldanha Bay and Langebaan Lagoon, South Africa*
Africa Africa’ (1997)4 given me the opportunity totackle thisOceanography, issue. UCT, and tocomments the onFlemish a draft ofFundthis article.for I amScientific also indebtedResearch to Dr. Johnin Belgium, Largier of whothe Departmenthave of around the coast ofSouth Africa.3 One ofthese regions is the West Coast extensive processes ofinvolving interested and affectedformulation parties ofin regions a vision for the coast of South Africa identified through Environmental Affairs and Tourism and supported by the United Sowman of the Department of Environmental and Geographical Sciences, UCT, for their Sustainable Coastal set Developmentout South Act.2 Africa’s Kingdom’s futureIts Departmentpolicy for startingon International coastal Development. pointmanagement It is in supposedwas ato (CMPP). new the This programme was launched by South Africa’s productMinistry ofof an extensive process of public participation and specialist with the changing socio-political environment in South Africarevealed the studies carried out through the Coastal Management Policy Programme offact, the present Act only appliesto areas belowthe highwater mark. coastal development through integrated coastal management.1 As inadequacya matter of the existing Seashore Act (1935) to achieve sustainable point for virtually the whole spectrum ofhuman activities and is subjectto increasing development demands and urbanisation. This concern coupled The ecologically varied 3200-km South African coastal zone is the focal AFRICA* Jan Schrijvers** TOWARDS INTEGRATED COASTAL MANAGEMENT FOR 1 1 Introduction SALDANHA ANDBAY LANGEBAAN LAGOON, SOUTH 3 3 See generally CMPP 2 irism in March 1999. 1 1 See further J Glazewski ‘Towards a coastal zone management Act for South * A draft Coastal Policy White Paper was submitted in March 1999 as the as submitted by the Policy Committee to the Minister of Environmental Affairs and See generally CMPP MSc (Gent) PhD(Gent) postdoctoral researcherICZM, University ofGent, Belgium. -

Vol. 4 APPENDIX F-3 Proofs of Submissions Received from I&Aps from Britannica Heights, St Helena Bay and the Petitions
FINAL ENVIRONMENTAL IMPACT ASSESSMENT REPORT Boulders Wind Farm Vol. 4 APPENDIX F-3 Proofs of submissions received from I&APs from Britannica Heights, St Helena Bay and the Petitions September 2019 DRAFT Environmental Impact Assessment Report TABLE OF CONTENTS Proofs of submissions received from I&APs ................................................................ 3 Britannica Heights Residents ......................................................................................... 3 Marx, Matthew ............................................................................................................................. 4 Jordaan, Deborah ....................................................................................................................... 9 Morley Robert ............................................................................................................................ 11 Anne and John Todd ................................................................................................................. 12 Doug Portsmouth ...................................................................................................................... 21 ST Helena Bay Residents ............................................................................................ 24 Smith Dereck ............................................................................................................................. 24 De Kock Colin........................................................................................................................... -

Bergrivier Municipality Spatial Development Framework: 2019 - 2024
Bergrivier Municipality Spatial Development Framework: 2019 - 2024 February 2019 Contact Professional Team Town Planning and Environmental Setplan Management [email protected] / janine@ Bergrivier Municipality setplan.com / [email protected] / [email protected] Werner Wagener Hannes Vermeulen Keenin Abrahams GLS Consulting [email protected] / [email protected] / [email protected] email: [email protected] / [email protected] call: Ursula Rigby 022 783 1112 / 022 913 6000 [email protected] visit: Bergrivier Municipal Offices Donovan Kirkwood Church Street 13 [email protected] Piketberg BERGRIVIER MUNICIPALITY Glossary of Terms & Abbreviations CBA - Critical Biodiversity Area PSG - Provincial Strategic Goal CRU - Community Residential Units RDP - Reconstruction and Development Programme CSIR - Council for Scientific and Industrial Research RSEP - Regional Socio-Economic Programme DEA&DP - Department of Environmental Affairs and RSIF - Regional Spatial Implementation Framework Development Planning SANRAL - SA National Road Agency DEDAT - Department of Economic Development and SBIDZ - Saldanha Bay Industrial Development Zone Tourism SDF - Spatial Development Framework DRDLR - Department of Rural Development and Land SDF - Spatial Development Goals Reform SEZ - Special Economic Zone DTPW - Department of Transport & Public Works SHI - Social Housing Institution DWS - Department of Water & Sanitation SHRA - Social Housing Regulatory Authority EIA - Environmental Impact Assessment SPLUMA - Spatial Planning and Land -

A Blurred Paradise : Insider and Outsider Perspectives on Paternoster
A Blurred Paradise: insider and outsider perspectives on Paternoster Alyson Karen Smith Student Number: SMTALI016 A minor dissertation submitted in partial fulfillment of the requirements for the award of the degree of Town Masters in Documentary Arts Department of Film and Media Studies Faculty of the HumanitiesCape University ofof Cape Town 2014 COMPULSORY DECLARATION This work has not beenUniversity previously submitted in whole, or in part, for the award of any degree. It is my own work. Each significant contribution to, and quotation in, this dissertation from the work, or works, of other people has been attributed, and has been cited and referenced. Signature: Date: Page 1 of 33 The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town A Blurred Paradise: insider and outsider perspectives on Paternoster Alyson Karen Smith ABSTRACT Paternoster is perpetually represented as being a tourist destination primarily through the use of imagery; yet this is often without interrogation of the primary narrative which when examined cracks in the visual façade surface. The overall objective of this research was to review the community conflicts through the use of an insider and outsider approach. This was achieved through using an insider qualitative, consensual, documentary style approach. Fieldwork, participant observation and interviews were used as inroads to the community and to enable the use of photography to explore existing narratives, why they exist and the possibility for alternative narratives. -

Integrated Report 2008
It all started in 1918 Oceana Group Limited Annual Report 2008 MISSION STATEMENT To be the leading fishing and commercial cold storage company in Africa offering: • a diversified range of fish products • strong brands • global sources of supply • empowerment, transformation and corporate social responsibility credentials resulting in superior returns to all stakeholders. CONTENTS 90TH ANNIVERSARY Mission statement IFC On 30 July 2008 Oceana celebrated its Group profile 1, 2 90th anniversary year of the founding of the Key group objectives 3 Financial and other significant highlights 4 company in Lambert’s Bay in 1918. Segmental report 5 Operating locations 6 Over the years, and despite the normal ups and Ownership profile 6 downs of a cyclical resource, the group’s core Group at a glance 7 fishing and cold storage operations have shown Directorate 8 consistent growth and stability. Oceana remains Executive committee 9 Chairman’s statement 10 committed to the principles of planning and Chief executive officer’s report 14 investing in the future and to the long-term Sustainability report 20 sustainability of its business. Risk management report 49 Audit committee and internal controls 50 Corporate governance 51 Remuneration report 52 Andrew Marshall Statistical and financial data 58 Oceana CEO Value added statement 60 Annual financial statements 61 Share analysis 97 Notice of annual general meeting 98 Curricula vitae 102 Form of proxy 103 Administration 105 Shareholders’ diary 105 Oceana Group Limited was incorporated in 1918. Its shares are listed on the Johannesburg Stock Exchange (JSE) and the Namibian Stock Exchange (NSX). It all started in 1918 Oceana Group Limited had its origins in the picturesque west coast town of Lambert’s Bay.