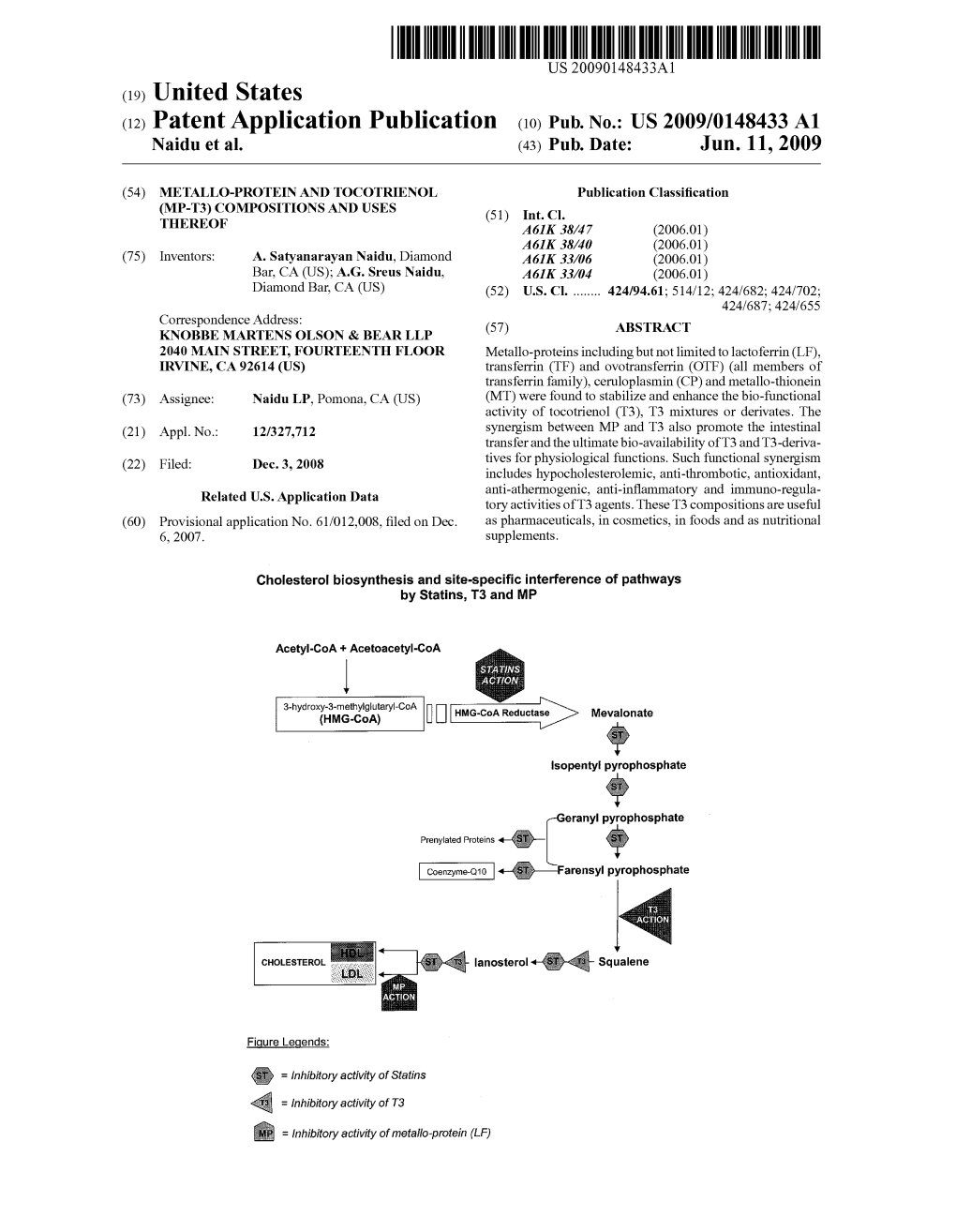
(12) Patent Application Publication (10) Pub. No.: US 2009/0148433 A1 Naidu Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flavonol Glycosides and Their Distribution in the Perianths of Gladiolus×Grandiflora Cultivars
Bull. Natl. Mus. Nat. Sci., Ser. B, 45(1), pp. 29–40, February 22, 2019 Flavonol Glycosides and their Distribution in the Perianths of Gladiolus×grandiflora Cultivars Tomoko Takemura1 and Tsukasa Iwashina2,* 1 United Graduate School of Agricultural Science, Tokyo University of Agriculture and Technology, Saiwai-cho, Fuchu 183–8509, Japan 2 Department of Botany, National Museum of Nature and Science, Amakubo 4–1–1, Tsukuba, Ibaraki 305–0005, Japan * E-mail: [email protected] (Received 16 October 2018; accepted 26 December 2018) Abstract Fifteen flavonol glycosides were isolated from the perianths of two Gladiolus×grandiflora cultivars “Ariake” and “Christmas” by various chromatography. Of their flavonols, eight glycosides were identified as kaempferol 3-O-sophoroside, 3-O-glucoside, 3-O-rutinoside and 3-O-glucosyl-(1→2)-rhamnoside, quercetin 3-O-rutinoside and myricetin 3-O-rutinoside, 3-O-glucoside and 3-O-rhamnoside by UV spectra, LC-MS, acid hydrolysis, 1H and 13C NMR, and TLC and HPLC comparisons with authentic samples. Other seven flavonols were characterized as kaempferol 3-O-pentosylglucoside, 3-O-rhamnosylrhamnosylglucoside and 3-O-glucosylrhamnoside, laricitrin 3-O-diglucoside, 3-O-rhamnosylglucoside and 3-O-rhamnosyl- hexoside, and syringetin 3-O-rhamnosylglucoside. Flavonol composition of the perianths of 89 Gladiolus×grandiflora cultivars including 12 purple, 13 reddish purple, 13 pink, 19 red, 2 orange, 16 white, 11 yellow and 3 green flower cultivars was surveyed by HPLC. Moreover, correlation between flower colors and flavonol -

Redalyc.Reactivity Indexes and O-H Bond Dissociation Energies of A
Journal of the Mexican Chemical Society ISSN: 1870-249X [email protected] Sociedad Química de México México Pérez-González, Adriana; Rebollar-Zepeda, Aida Mariana; León-Carmona, Jorge Rafael; Galano, Annia Reactivity Indexes and O-H Bond Dissociation Energies of a Large Series of Polyphenols: Implications for their Free Radical Scavenging Activity Journal of the Mexican Chemical Society, vol. 56, núm. 3, julio-septiembre, 2012, pp. 241-249 Sociedad Química de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=47524533003 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative J. Mex. Chem. Soc. 2012, 56(3), 241-249 ArticleReactivity Indexes and O-H Bond Dissociation Energies of a Large Series of Polyphenols: Implications ©for 2012, their SociedadFree Radical Química de México241 ISSN 1870-249X Reactivity Indexes and O-H Bond Dissociation Energies of a Large Series of Polyphenols: Implications for their Free Radical Scavenging Activity Adriana Pérez-González, Aida Mariana Rebollar-Zepeda, Jorge Rafael León-Carmona, and Annia Galano∗ Departamento de Química. Universidad Autónoma Metropolitana-Iztapalapa. San Rafael Atlixco 186, Col. Vicentina. Iztapalapa. C. P. 09340. México D. F. México. [email protected] To Professor José Luis Gázquez Mateos for being a great example and a constant motivation. We sincerely thank him for sharing his kindness and knowledge with all of us. Received September 19, 2011; accepted February 20, 2012 Abstract. Several chemical descriptors have been evaluated for thirty Resumen. -

Anti-Cov-2 Spike Protein Directed Druggable Inhibitors
OPEN ACCESS ECOCYCLES Scientific journal of the ISSN 2416-2140 European Ecocycles Society Ecocycles, Vol. 6, No. 2, pp. 38-45 (2020) DOI: 10.19040/ecocycles.v6i2.176 ORIGINAL PAPER Effective natural food complements: anti-CoV-2 spike protein directed druggable inhibitors Oláh, Zoltán1-3*; Ökrész, László1; Török, Ibolya1; Pestenácz, Anikó1; Harkai, Anikó1; Kocsis, Éva1-3 1Acheuron Ltd., Szeged, Hungary 2Forget-Me-Not B2B Ltd., Szeged, Hungary 3 Vásárhely’s Landscaping (VATES) Folks- School Society, Hódmezővásárhely, Hungary *corresponding author, e-mail: [email protected] Abstract – There is a number of photosynthetically produced small molecules that have previously been validated through SARS- CoV spike protein interaction assays for selectivity and effectivity in our database. Our specialty database, the AVIRA-DB, has been built from scientific papers that published results regarding selective & effective CoV-2 spike protein binding inhibitors that prevent virus binding to the Angiotensin Converting Enzyme type 2 (ACE2). These data have been accumulated since 2003, the time of the first well documented coronavirus pandemic. To develop our anti-viral nutraceutical capsule we favoured small molecules (Mw <1000 Dalton) from edible plant parts that are enriched in experimentally evaluated coronavirus inhibitors. From this “AVIRA-DB” we screened for local culture varieties of vegetables and spices that are enriched in the anti-viral hits. Thus, AVIRA is the first knowledge-based nutraceutical composition that was validated by selective anti-CoV-2’s spike protein assays, performed in silico, -vitro & -vivo. From Chemo- & Bio-text-mined meta-data from literature and patents resulted in druggable flavonoids and flavonols, which were validated as anti-CoV-2 spike protein directed small molecules that are preventing the binding of the virus to ACE2. -

Comprehensive Chemical and Sensory Assessment of Wines Made from White Grapes of Vitis Vinifera Cultivars Albillo Dorado And
foods Article Comprehensive Chemical and Sensory Assessment of Wines Made from White Grapes of Vitis vinifera Cultivars Albillo Dorado and Montonera del Casar: A Comparative Study with Airén José Pérez-Navarro 1 , Pedro Miguel Izquierdo-Cañas 2,3, Adela Mena-Morales 2, Juan Luis Chacón-Vozmediano 2, Jesús Martínez-Gascueña 2, Esteban García-Romero 2, 1, 1, Isidro Hermosín-Gutiérrez y and Sergio Gómez-Alonso * 1 Regional Institute for Applied Scientific Research (IRICA), University of Castilla-La Mancha, Av. Camilo José Cela, 10, 13071 Ciudad Real, Spain; [email protected] 2 Instituto Regional de Investigación y Desarrollo Agroalimentario y Forestal de Castilla-La Mancha (IRIAF), Ctra. Albacete s/n, 13700 Tomelloso, Spain; [email protected] (P.M.I.-C.); [email protected] (A.M.-M.); [email protected] (J.L.C.-V.); [email protected] (J.M.-G.); [email protected] (E.G.-R.) 3 Parque Científico y Tecnológico de Castilla-La Mancha, Paseo de la Innovación 1, 02006 Albacete, Spain * Correspondence: [email protected] In memoriam. y Received: 17 August 2020; Accepted: 10 September 2020; Published: 12 September 2020 Abstract: The ability to obtain different wines with a singular organoleptic profile is one of the main factors for the wine industry’s growth, in order to appeal to a broad cross section of consumers. Due to this, white wines made from the novel grape genotypes Albillo Dorado and Montonera del Casar (Vitis vinifera L.) were studied and compared to the well-known Airén at two consecutive years. Wines were evaluated by physicochemical, spectrophotometric, high-performance liquid chromatography–diode array detection–mass spectrometry, gas chromatography–mass spectrometry and sensory analyses. -

Flavonoids from Artemisia Annua L. As Antioxidants and Their Potential Synergism with Artemisinin Against Malaria and Cancer
Molecules 2010, 15, 3135-3170; doi:10.3390/molecules15053135 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer 1, 2 3 4 Jorge F.S. Ferreira *, Devanand L. Luthria , Tomikazu Sasaki and Arne Heyerick 1 USDA-ARS, Appalachian Farming Systems Research Center, 1224 Airport Rd., Beaver, WV 25813, USA 2 USDA-ARS, Food Composition and Methods Development Lab, 10300 Baltimore Ave,. Bldg 161 BARC-East, Beltsville, MD 20705-2350, USA; E-Mail: [email protected] (D.L.L.) 3 Department of Chemistry, Box 351700, University of Washington, Seattle, WA 98195-1700, USA; E-Mail: [email protected] (T.S.) 4 Laboratory of Pharmacognosy and Phytochemistry, Ghent University, Harelbekestraat 72, B-9000 Ghent, Belgium; E-Mail: [email protected] (A.H.) * Author to whom correspondence should be addressed; E-Mail: [email protected]. Received: 26 January 2010; in revised form: 8 April 2010 / Accepted: 19 April 2010 / Published: 29 April 2010 Abstract: Artemisia annua is currently the only commercial source of the sesquiterpene lactone artemisinin. Since artemisinin was discovered as the active component of A. annua in early 1970s, hundreds of papers have focused on the anti-parasitic effects of artemisinin and its semi-synthetic analogs dihydroartemisinin, artemether, arteether, and artesunate. Artemisinin per se has not been used in mainstream clinical practice due to its poor bioavailability when compared to its analogs. In the past decade, the work with artemisinin-based compounds has expanded to their anti-cancer properties. -

Integrative Itraq-Based Proteomic and Transcriptomic Analysis Reveals
Ye et al. Horticulture Research (2021) 8:157 Horticulture Research https://doi.org/10.1038/s41438-021-00591-2 www.nature.com/hortres ARTICLE Open Access Integrative iTRAQ-based proteomic and transcriptomic analysis reveals the accumulation patterns of key metabolites associated with oil quality during seed ripening of Camellia oleifera Zhouchen Ye1,JingYu1,WupingYan1,JunfengZhang1, Dongmei Yang1, Guanglong Yao1, Zijin Liu1, Yougen Wu 1 and Xilin Hou 2 Abstract Camellia oleifera (C. oleifera) is one of the four major woody oil-bearing crops in the world and has relatively high ecological, economic, and medicinal value. Its seeds undergo a series of complex physiological and biochemical changes during ripening, which is mainly manifested as the accumulation and transformation of certain metabolites closely related to oil quality, especially flavonoids and fatty acids. To obtain new insights into the underlying molecular mechanisms, a parallel analysis of the transcriptome and proteome profiles of C. oleifera seeds at different maturity levels was conducted using RNA sequencing (RNA-seq) and isobaric tags for relative and absolute quantification (iTRAQ) complemented with gas chromatography-mass spectrometry (GC-MS) data. A total of 16,530 transcripts and 1228 proteins were recognized with significant differential abundances in pairwise comparisons of samples at various developmental stages. Among these, 317 were coexpressed with a poor correlation, and most were involved in metabolic processes, including fatty acid metabolism, α-linolenic acid metabolism, and glutathione metabolism. In addition, the content of total flavonoids decreased gradually 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; with seed maturity, and the levels of fatty acids generally peaked at the fat accumulation stage; these results basically agreed with the regulation patterns of genes or proteins in the corresponding pathways. -

In Silico, in Vitro and in Vivo Memory Enhancing Activity
IN SILICO, IN VITRO AND IN VIVO MEMORY ENHANCING ACTIVITY OF CERTAIN COMMERCIALLY AVAILABLE FLAVONOIDS IN SCOPOLAMINE AND ALUMINIUM-INDUCED LEARNING IMPAIRMENT IN MICE Thesis submitted to The Tamil Nadu Dr. M.G.R. Medical University, Chennai for the award of the degree of DOCTOR OF PHILOSOPHY in PHARMACY Submitted by A. MADESWARAN, M. Pharm., Under the guidance of Dr. K. ASOK KUMAR, M. Pharm., Ph.D. College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, Coimbatore – 641 044, Tamil Nadu, India. JUNE 2017 Certificate This is to certify that the Ph.D. dissertation entitled “IN SILICO, IN VITRO AND IN VIVO MEMORY ENHANCING ACTIVITY OF CERTAIN COMMERCIALLY AVAILABLE FLAVONOIDS IN SCOPOLAMINE AND ALUMINIUM- INDUCED LEARNING IMPAIRMENT IN MICE” being submitted to The Tamil Nadu Dr. M.G.R. Medical University, Chennai, for the award of degree of DOCTOR OF PHILOSOPHY in the FACULTY OF PHARMACY was carried out by Mr. A. MADESWARAN, in College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, Coimbatore, under my direct supervision and guidance to my fullest satisfaction. The contents of this thesis, in full or in parts, have not been submitted to any other Institute or University for the award of any degree or diploma. Dr. K. Asok Kumar, M.Pharm., Ph.D. Professor & Head, Department of Pharmacology, College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, Coimbatore, Tamil Nadu - 641 044. Place: Coimbatore – 44. Date: Certificate This is to certify that the Ph.D. dissertation entitled “IN SILICO, IN VITRO AND IN VIVO MEMORY ENHANCING ACTIVITY OF CERTAIN COMMERCIALLY AVAILABLE FLAVONOIDS IN SCOPOLAMINE AND ALUMINIUM- INDUCED LEARNING IMPAIRMENT IN MICE” being submitted to The Tamil Nadu Dr. -

Molecules 2010, 15, 3135-3170; Doi:10.3390/Molecules15053135
Molecules 2010, 15, 3135-3170; doi:10.3390/molecules15053135 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer 1, 2 3 4 Jorge F.S. Ferreira *, Devanand L. Luthria , Tomikazu Sasaki and Arne Heyerick 1 USDA-ARS, Appalachian Farming Systems Research Center, 1224 Airport Rd., Beaver, WV 25813, USA 2 USDA-ARS, Food Composition and Methods Development Lab, 10300 Baltimore Ave,. Bldg 161 BARC-East, Beltsville, MD 20705-2350, USA; E-Mail: [email protected] (D.L.L.) 3 Department of Chemistry, Box 351700, University of Washington, Seattle, WA 98195-1700, USA; E-Mail: [email protected] (T.S.) 4 Laboratory of Pharmacognosy and Phytochemistry, Ghent University, Harelbekestraat 72, B-9000 Ghent, Belgium; E-Mail: [email protected] (A.H.) * Author to whom correspondence should be addressed; E-Mail: [email protected]. Received: 26 January 2010; in revised form: 8 April 2010 / Accepted: 19 April 2010 / Published: 29 April 2010 Abstract: Artemisia annua is currently the only commercial source of the sesquiterpene lactone artemisinin. Since artemisinin was discovered as the active component of A. annua in early 1970s, hundreds of papers have focused on the anti-parasitic effects of artemisinin and its semi-synthetic analogs dihydroartemisinin, artemether, arteether, and artesunate. Artemisinin per se has not been used in mainstream clinical practice due to its poor bioavailability when compared to its analogs. In the past decade, the work with artemisinin-based compounds has expanded to their anti-cancer properties. -

Advances in the Knowledge of Olive Oil Phenolic Compounds. from Biotransformations After Their Intake to Their Metabolic Fate for Exerting Biological Activities
Nom/Logotip de la Universitat on s’ha llegit la tesi Advances in the knowledge of olive oil phenolic compounds. From biotransformations after their intake to their metabolic fate for exerting biological activities Maria del Carmen López de las Hazas Mingo http://hdl.handle.net/10803/402402 ADVERTIMENT. L'accés als continguts d'aquesta tesi doctoral i la seva utilització ha de respectar els drets de la persona autora. Pot ser utilitzada per a consulta o estudi personal, així com en activitats o materials d'investigació i docència en els termes establerts a l'art. 32 del Text Refós de la Llei de Propietat Intel·lectual (RDL 1/1996). Per altres utilitzacions es requereix l'autorització prèvia i expressa de la persona autora. En qualsevol cas, en la utilització dels seus continguts caldrà indicar de forma clara el nom i cognoms de la persona autora i el títol de la tesi doctoral. No s'autoritza la seva reproducció o altres formes d'explotació efectuades amb finalitats de lucre ni la seva comunicació pública des d'un lloc aliè al servei TDX. Tampoc s'autoritza la presentació del seu contingut en una finestra o marc aliè a TDX (framing). Aquesta reserva de drets afecta tant als continguts de la tesi com als seus resums i índexs. ADVERTENCIA. El acceso a los contenidos de esta tesis doctoral y su utilización debe respetar los derechos de la persona autora. Puede ser utilizada para consulta o estudio personal, así como en actividades o materiales de investigación y docencia en los términos establecidos en el art. -

Analysis of Antioxidant Activity and Flavonoids Metabolites in Peel and Flesh of Red-Fleshed Apple Varieties
Supplementary Analysis of Antioxidant Activity and Flavonoids Metabolites in Peel and Flesh of Red-Fleshed Apple Varieties Xiang Zhang 1,2,†, Jihua Xu 1,3,†, Zhaobo Xu 4, Xiaohong Sun 1,3, Jun Zhu 1,2 and Yugang Zhang 1,2,* 1 Qingdao Key Laboratory of Genetic Development and Breeding in Horticultural Plants, Qingdao Agricultural University, Qingdao 266109, China; [email protected] (X.Z.); [email protected] (J.X.); [email protected] (X.S.); [email protected] (J.Z.) 2 College of Horticulture, Qingdao Agricultural University, Qingdao 266109, China 3 College of Life Sciences, Qingdao Agricultural University, Qingdao 266109, China 4 Qingdao Agriculture Technology Extension Center, Qingdao 266071, China; [email protected] (Z.X.) * Correspondence: [email protected]; Tel.: + 86-0532-589-57740 † These authors contributed equally to this work. Received: 17 March 2020; Accepted: 21 April 2020; Published: Figure S1. Principle component analysis (PCA) score plot, orthogonal partial least squares- discriminant analysis (OPLS-DA) score plot and permutation test in four comparison groups. (A,B) PCA in DHPM vs. XJ4PM and DHFM vs. XJ4FM. (C,D) OPLS-DA in DHPM vs. XJ4PM and DHFM vs. XJ4FM. (E,F) Permutation test in DHPM vs. XJ4PM and DHFM vs. XJ4FM. Molecules 2019, 24, x; doi: FOR PEER REVIEW www.mdpi.com/journal/molecules Molecules 2019, 24, x FOR PEER REVIEW 2 of 5 1 Table S1. The differential metabolites in both DHPM vs. XJ4PM and DHFM vs. XJ4FM. Content Fold change Combination name Metabolite name VIP Grouping of specific metabolites DH XJ4 (XJ4PM/DHPM; XJ4FM/DHFM) ANTHOCYANIN DHPM vs. -

Bryonia Dioica Aqueous Extract Induces Apoptosis and G2/M Cell Cycle Arrest in MDA‑MB 231 Breast Cancer Cells
MOLECULAR MEDICINE REPORTS 20: 73-80, 2019 Bryonia dioica aqueous extract induces apoptosis and G2/M cell cycle arrest in MDA‑MB 231 breast cancer cells BACHIR BENARBA1, ALMAHY ELMALLAH2 and ATANASIO PANDIELLA3 1Laboratory Research on Biological Systems and Geomatics, Faculty of Nature and Life, University of Mascara, Mascara 29000, Algeria; 2Zoology Department, Faculty of Science Beni-Suef University, Beni‑Suef 62511, Egypt; 3Instituto de Biología Molecular y Celular del Cáncer, CSIC-CIBERONC-Universidad de Salamanca, 37001 Salamanca, Spain Received June 29, 2018; Accepted March 1, 2019 DOI: 10.3892/mmr.2019.10220 Abstract. Bryonia dioica Jacq. is a climbing perennial herb Introduction with tuberous roots which is widely used in traditional medi- cine in Algeria for the treatment of cancer. The present study Breast cancer, considered heterogeneous cancer, both biologi- aimed to evaluate the apoptogenic activity and phytochemical cally and molecularly, is the most common malignancy in composition of the aqueous extract of B. dioica roots growing women worldwide (1). Breast cancer still remains the first in Algeria. The cytotoxic effect of B. dioica aqueous decoc- cause of cancer-related deaths among women, especially in tion against breast cancer MDA‑MB-231 cells was evaluated developing countries in which 60% of breast cancer-related by an MTT assay. Apoptosis induction was assessed by an deaths are reported (2). In spite of being treated by different Annexin V‑fluorescien iosthiocyanate assay. Propidium iodide therapeutic approaches including chemotherapy, radiotherapy, staining of cell DNA was used to assess the effects on the cell surgery, hormone therapy and other targeted therapies, breast cycle. -
Treasure from Garden: Bioactive Compounds of Buckwheat
Food Chemistry 335 (2021) 127653 Contents lists available at ScienceDirect Food Chemistry journal homepage: www.elsevier.com/locate/foodchem Treasure from garden: Bioactive compounds of buckwheat T Md. Nurul Hudaa,1, Shuai Lua,1, Tanzim Jahanb, Mengqi Dingc, Rintu Jhaa, Kaixuan Zhanga, ⁎ Wei Zhangd, Milen I. Georgieve,f, Sang Un Parkc, Meiliang Zhoua, a Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China b Department of Biological Science, Faculty of Science, King Abdulaziz University, Jeddah 80208, Saudi Arabia c Department of Crop Science, College of Agriculture & Life Sciences, Chungnam National University, Yuseong-gu, Daejeon 305-754, Republic of Korea d College of Food Science and Technology, Hebei Agricultural University, Baoding 071001, China e Laboratory of Metabolomics, The Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences, Plovdiv, Bulgaria f Center of Plant Systems Biology and Biotechnology, Plovdiv, Bulgaria ARTICLE INFO ABSTRACT Keywords: Buckwheat is a gluten-free crop under the family Polygonaceae abundant with beneficial phytochemicals that Buckwheat provide significant health benefits. It is cultivated and adapted in diverse ecological zones all over the world. D-chiro-inositol Recently its popularity is expanding as a nutrient-rich healthy food with low-calories. The bioactive compounds Flavonoids in buckwheat are flavonoids (i.e., rutin, quercetin, orientin, isoorientin, vitexin, and isovitexin), fatty acids, Nutritional value polysaccharides, proteins, and amino acids, iminosugars, dietary fiber, fagopyrins, resistant starch, vitamins, and Rutin minerals. Buckwheat possesses high nutritional value due to these bioactive compounds. Additionally, several essential bioactive factors that have long been gaining interest because these compounds are beneficial for healing and preventing several human diseases.