KVS Technologies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
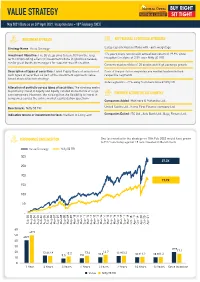
Value Strategy Note 04-05-2021
VALUE STRATEGY May 2021 (Data as on 30th April 2021. Inception date – 18th February 2003) INVESTMENT APPROACH KEY FEATURES & PORTFOLIO ATTRIBUTES Strategy Name: Value Strategy Large cap oriented portfolio with ~66% weightage Investment Objective: The Strategy aims to benefit from the long 17+ years track record with annualized return of 19.9% since term compounding effect on investments done in good businesses, inception (an alpha of 2.8% over Nifty 50 TRI) run by great business managers for superior wealth creation. Concentrated portfolio of 25 stocks with high earnings growth Description of types of securities: Listed Equity Basis of selection of Each of the portfolio companies are market leaders in their such types of securities as part of the investment approach: value respective segments based stock selection strategy Index agnostic: ~71% away from benchmark Nifty 50 Allocation of portfolio across types of securities: The strategy seeks to primarily invest in Equity and Equity-related instruments of large cap companies. However, the strategy has the flexibility to invest in PORTFOLIO ACTIONS IN LAST 6 MONTHS companies across the entire market capitalization spectrum Companies Added: Mahindra & Mahindra Ltd., Benchmark: Nifty 50 TRI United Spirits Ltd., Home First Finance company Ltd. Indicative tenure or investment horizon: Medium to Long term Companies Exited: ITC Ltd., Axis Bank Ltd., Bajaj Finserv Ltd. PERFORMANCE SINCE INCEPTION One lac invested in the strategy on 18th Feb 2003 would have grown to ` 27 lacs today against 18 lacs -

August 5, 2020 National Stock Exchange of India
Registered Office Address: Mindtree Limited Global Village, RVCE Post, Mysore Road, Bengaluru – 560059, Karnataka, India. Corporate identity Number (CIN): L72200KA1999PLC025564 E-mail : [email protected] Ref: MT/STAT/CS/20-21/111 August 5, 2020 BSE Limited National Stock Exchange of India Limited Phiroze Jeejeebhoy Towers, Exchange Plaza, Dalal Street, Bandra Kurla Complex, Bandra East, Mumbai 400 001. Mumbai 400 051. Dear Sirs, Subject: Press Release - Mindtree Appoints Venu Lambu as President of Global Markets This is to inform you that, the Company proposes to make a press release on the following topic: “Mindtree Appoints Venu Lambu as President of Global Markets” A copy of the press release to be distributed to the media is enclosed herewith. Please take the above intimation on records. Thanking you. Yours sincerely, for Mindtree Limited Vedavalli S Company Secretary Encl: as above _____________________________________________________________________________________ Mindtree Limited Global Village T + 91 80 6706 4000 RVCE Post, Mysore Road F +91 80 6706 4100 Bengaluru – 560059 W www.mindtree.com Mindtree Appoints Venu Lambu as President of Global Markets Veteran technology services executive will focus on accelerating revenue growth Warren (NJ) and Bangalore (India) – August 05, 2020 – Mindtree, the leading digital transformation and technology services company, today announced the appointment of Venu Lambu as President of Global Markets, a role strategically oriented towards accelerating Mindtree’s revenue growth. Based in London, Venu will be responsible for formulating strategic direction and business development for Mindtree businesses across all industry segments. “Venu has the ideal combination of leadership acumen, energy, and experience to help drive our aggressive plans to further scale the business,” said Debashis Chatterjee, Chief Executive Officer and Managing Director of Mindtree. -

Business Services HCL Technologies Limited
HCL Technologies Ltd. - Business Services │Company Profile HCL Technologies Limited pioneered third party BPO (Business Process Outsourcing) industry in India by launching HCL Business Services (formerly HCL BPO Services) in 2001. HCL Business Services provides 24X7, multi-channel and multi-lingual support in 8 European languages. Over 11,500 professionals operate out of 26 delivery centers across US, UK, APAC, Europe and America. HCL Business Services focuses on key industry segments such as Financial Services, Health Care Services, Telecom, Energy & Utility, Public Services, Manufacturing, Retail & CPG, Media and Publishing, and Logistics. In addition to providing vertical industry specific solutions, HCL Business Services provides the following cross industry horizontal solutions: Finance & Accounting Outsourcing, Human Resources Outsourcing, Customer Relationship Management, Knowledge Process Outsourcing, Technical Support Services and Supply Chain Management (SCM). HCL also pioneered the multi-country delivery and the platform business model. With over a decade of industry experience, and Fortune 500/Fortune Global 500 customers, HCL today pursues a revolutionary maturity level where a new form of BPO called ‘Transformational BPO’ is evolving which involves Full Process and Multi-Process outsourcing. This strategic decision to evolve from the traditional contact center model to the more holistic Business Services model is to effectively address the evolving requirements and expectations of BPO customers worldwide. HCL leverages its -

1 AGENDA for 111Th UNIT APPROVAL Committees
111th UAC Meeting (29 .11.2019), VISAKHAPATNAM AGENDA FOR 111th UNIT APPROVAL COMMITTEEs MEETING FOR THE PRIVATE SEZs OF ANDHRA PRADESH REGION TO BE HELD ON 29.11.2019 AT 11.00 A.M. AT CONFERENCE HALL, ADMINISTRATIVE BUILDING, VISAKHAPATNAM SPECIAL ECONOMIC ZONE, DUVVADA, VISAKHAPATNAM. Sl. Agenda - PRIVATE SEZS in Andhra Pradesh Page No No. 1 Agenda Item No.01: Ratification of the minutes of the meeting held on 25.09.2019 2 2 Agenda Item No. 02: Proposal of M/s. HCL Technologies Ltd for setting up of a u nit in 2-4 M/s. HCL Technologies Ltd SEZ at Sy.No.20/3, Kesarapalli vill. NH 5, Gannavaram Mandal, Vijayawada, Andhra Pradesh 3 Agenda Item No.03: Request of M/s.Honour Lab Limited in PlotNo.4, Hetero infrastructure 4-5 Ltd-SEZ,. Nakkapally for inclusion of additional products in the LoA. 4 Agenda Item No.04: M/s. Natco Pharma Limited, a unit in Ramky Pharma SEZ, Parawada, 5-6 Visakhapatnam for inclusion of additional products in the LoA. 5 Agenda No. 05: Request of M/s. Biocon Limited, Co Developer of M/s Ramky Pharma City 6-7 SEZ, Parawada, Visakhapatnam for permission for procure material without payment of duty on the basis of approval of Authorized operations 1 111th UAC Meeting (29 .11.2019), VISAKHAPATNAM AGENDA FOR 111th UNIT APPROVAL COMMITTEEs MEETING FOR THE SEZs LOCATED IN PRIVATE SEZs OF ANDHRA PRADESH REGION TO BE HELD ON 29.11.2019 AT 11.00 A.M. AT CONFERENCE HALL, ADMINISTRATIVE BUILDING, VISAKHAPATNAM SPECIAL ECONOMIC ZONE, DUVVADA, VISAKHAPATNAM. -

Everest Group's PEAK Matrix for Banking AO
TM Topic: Everest Group’s PEAK Matrix for Banking AO Service Providers Focus on HCL Technologies July 2015 Copyright © 2015 Everest Global, Inc. This document has been licensed for exclusive use and distribution by HCL Technologies Background and scope of the research Background of the research Rise of digital consumers, need for business agility, growing costs of regulatory compliance, and threat from non-banking competitors in areas such as payments were the key challenges faced by banks in 2014. Banks are aligning their technology priorities around cost containment, efficiency, risk & compliance management, and transformation. Banks continued to invest in development of specific applications for regulatory compliance, risk management, channel-specific initiatives (social, mobile, etc.), and customer analytics The service provider landscape is intensifying as providers ramp up their capabilities, invest in innovative technologies, form alliances, and acquire strategic targets to address the growing and complex application services needs of banks and financial institutions In this research, we analyze the capabilities of 30 leading AO service providers, specific to the global banking sector. These providers were mapped on the Everest Group Performance | Experience | Ability | Knowledge (PEAK) Matrix, which is a composite index of a range of distinct metrics related to each provider’s capability and market success. Additionally, Everest Group has also profiled the capabilities of these 30 service providers in detail including a comprehensive picture of their service suite, scale of operations, domain investments, delivery locations, and buyer satisfaction specific to banking AO services This document focuses on HCL Technologies’ banking AO experience and includes: Overview of the banking AO service provider landscape Everest Group’s assessment of HCL Technologies’ delivery capability and market success on PEAK matrix Detailed banking AO profile for HCL Technologies Copyright © 2015, Everest Global, Inc. -
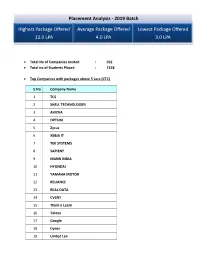
Placement Analysis - 2019 Batch
Placement Analysis - 2019 Batch Highest Package Offered Average Package Offered Lowest Package Offered 12.0 LPA 4.0 LPA 3.0 LPA Total No of Companies Invited : 502 Total no of Students Placed : 1328 Top Companies with packages above 5 Lacs (CTC) S.No Company Name 1 TCS 2 SHELL TECHNOLOGIES 3 AVIZVA 4 OPTUM 5 Zycus 6 XEBIA IT 7 TEK SYSTEMS 8 SAPIENT 9 MANN INDIA 10 HYUNDAI 11 YAMAHA MOTOR 12 RELIANCE 13 REAL DATA 14 CVENT 15 Think n Learn 16 Tolexo 17 Google 18 Dyson 19 United Lex 20 WIPRO WASE 21 HSBC 22 Accolite 23 Novanet 24 Foresight 25 Blue Star 26 RMS 27 Samsung 28 Amazon 29 Keyence 30 Veris Top Payers /Dream Companies Company Name CTC (LPA) INDIAN AIR FORCE 12 GOOGLE 12 HSBC 12 SIRION LABS 12 NATIONAL INSTRUMENTS 12 SHELL TECHNOLOGIES 10 THINK N LEARN 10 ACCOLITE 10 VMWARE 9.12 TCS (CODEVITA) 7.5 HYUNDAI 7.5 NOVANET 7 ZYCUS 6.5 XEBIA IT 6 MANN INDIA TECHNOLOGIES 6 RELIANCE 6 REAL DATA 6 FORESIGHT 6 BLUE STAR 6 SAMSUNG 6 AMAZON 6 KEYENCE 6 WIPRO WASE 5.57 OPTUM 5.4 SAPIENT 5.3 AVIZVA 5.25 DYSON 5.07 YAMAHA 5 CVENT 5 TOLEXO 5 UNITED LEX 5 PFIZER 5 LAMBORGINI 5 Top Recruiters Company Name Final Placed ACCENTURE 309 TCS 120 WIPRO TECHNOLOGIES 70 THINK N LEARN 40 ICONMA 39 NEC TECHNOLOGIES 29 AMAZON 52 FLIPKART 25 RELIANCE 23 MANN INDIA TECHNOLOGIES 21 SOPRA STERIA 20 HEXAWARE 16 HCL TECHNOLOGIES 23 SAMSUNG 28 ERICSSON 15 NTT DATA 33 Top IT Companies: S.No Company Name 1 NEC 2 TATA TECHNOLOGIES 3 WIPRO 4 ACCENTURE 5 HEXAWARE 6 OPTUM 7 NIIT 8 GRAPECITY 9 TCS 10 MPHASIS 11 SAPIENT 12 NEWGEN 13 ACCENTURE 14 Gemalto 15 Google 16 Genpact -

ISB-Biocon Certificate Program in Business Analytics Profile Book 2013
ISB-Biocon Certificate Program in Business Analytics Profile Book 2013 - 14 Class Profile The programme attracts exceptionally talented and vibrant group of individuals from diverse industries. The class composition of the 2013-14 batch is given below. Education Background Work Experience Industry Clusters • BE/ B.Tech - 44% • MCA - 5% • 0-4 years - 23% • Analytic Firms - 21% • BFSI - 3% • BE/ B.Tech/ MBA - 17% • ME/ M.Tech - 3% • 4-8 years - 33% • Indian IT - 23% • Captives - 4% • M.Sc. (Maths) - 5% • Others Grad. - 3% • 8-12 years - 22% • MNC IT - 26% • KPO - 7% • Grad + MBA - 22% • Ph.D. - 1% • 12-18 years - 20% • Telecom - 6% • FMCG - 4% • Above 18 years - 2% • Auto - 35 • Real Estate - 3% 60% of the Batch have a BE/ B.Tech Degree 56% of the Batch lies between 0 - 8 years 71% of the batch comes from three clusters - 55% of the Batch has Post Graduate Degree Analytics Firms, Indian IT Companies & MNC IT Companies Abdul Afzal Abhishek Manjrekar Amandeep Singh Amit Pradhan SAP HANA Technical Architect, Attune Consulting Deputy Manager - Marketing (Strategy & Analysis) Portfolio Manager (IT), Ford Motor Company Sr. Group Manager / AVP - Quality, WNS Global Services H&R Johnson(India), Mumbai Responsible for managing portfolio of IT Projects aligned to Current Role Current Role Ford Customer Services Division. This includes delivery, general Current Role SAP HANA / BI competency head & financial management. Additionally responsible for setting Master Black Belt - Leading the Process Excellence, Analytics, and Initiation & Analysis of the Dealer Management Group, Mason up Analytics Practice for Ford in India & designing Analytics Quality Assurance for a clients in the Insurance vertical Previous Assignments Insurance Program, Business Process Re-Engineering, Green Tiles Competency Framework. -

Mixed Quarter, Remain Selective Sector Update
IT Mixed quarter, remain selective Sector Update During April-June 2019 quarter, tier-1 Indian IT companies clocked Q1FY2020 Results Review mixed revenue growth, while EBIT margins declined across companies Sector: IT Services sequentially. Constant currency (CC) revenue growth for TCS, Infosys and HCL Tech remained strong at 10.6%, 12.4% and 17%, y-o-y Sector View: Neutral respectively. HCL Tech impressed with strong CC revenue growth of 4.2%, led by large deal ramp-ups, while TCS and Wipro missed our revenue estimates on soft growth in the BFSI vertical, as was the case with many other IT firms as well. However, most companies saw strong deal wins continue and a higher digital market share along with robust Our coverage universe y-o-y growth. Except TCS, elevated attrition remained a concern for most of the companies. Margins declined owing to wage revision, a rise CMP PT Companies Reco. (Rs) (Rs) in visa costs, transition costs for large deals, subcontracting costs and a stronger rupee. Most companies broadly maintained revenue growth HCL Tech 1,078 Buy 1,250 guidance for FY2020E, except Infosys, which raised its guidance despite a weak macro environment. Infosys 795 Buy 840 Outlook: L&T Infotech 1,593 Positive 1698 Cautious outlook on BFSI amid macro uncertainties, maintain Neutral L&T Tech 1,573 Positive 1847 stance: As spending by clients with exposure to capital markets Mastek 316 Positive 513 (especially in the US) and large European banks moderates and falling Persistent 564 Hold 650 interest rate in North America, we believe that growth in the BFSI vertical will remain weak in FY2020E. -

Mainframe Services & Solutions
Mainframe Services & Solutions A research report U.S. 2021 comparing provider strengths, challenges and competitive differentiators Quadrant Report Customized report courtesy of: April 2021 ISG Provider Lens™ Quadrant Report | April 2021 Section Name About this Report Information Services Group Inc. is solely responsible for the content of this report. ISG Provider Lens™ delivers leading-edge and actionable research studies, reports Unless otherwise cited, all content, including illustrations, research, conclusions, and consulting services focused on technology and service providers’ strengths and assertions and positions contained in this report were developed by, and are the sole weaknesses and how they are positioned relative to their peers in the market. These property of Information Services Group Inc. reports provide influential insights accessed by our large pool of advisors who are actively advising outsourcing deals as well as large numbers of ISG enterprise clients The research and analysis presented in this report includes research from the ISG who are potential outsourcers. Provider Lens™ program, ongoing ISG Research programs, interviews with ISG advisors, briefings with services providers, and analysis of publicly available market information For more information about our studies, please email [email protected], from multiple sources. The data collected for this report represents information that call +49 (0) 561-50697537, or visit ISG Provider Lens™ under ISG Provider Lens™. ISG believes to be current as of February 2021, for providers who actively participated as well as for providers who did not. ISG recognizes that many mergers and acquisitions have taken place since that time, but those changes are not reflected in this report. -

Application Management and Development - Takeaways from IBM Services
Application Management and Development - Takeaways from IBM Services FEATURING RESEARCH FROM FORRESTER The Forrester Wave™: Applications Management And Digital Operations Services, Q4 2017 2 Application Management and Development - Takeaways from IBM Services Today’s enterprises are continuously challenged to keep pace in a business world increasingly populated by nimble startups and born-digital companies, especially those that achieve formidable scale very fast. These incumbent enterprises once relied on their IT cores as a source of competitive advantage. Now, those same IT cores are increasingly making it difficult for them to adapt to a “new normal” in terms of speed, efficiency and resilience: Speed: Traditional release cycles took months. With the help of agile development methods, and tightly integrated team structures, today’s leading businesses are able to deploy with hyper-frequency, allowing them to introduce new features and capabilities on a continuous basis. Efficiency: Through greater business integration, automation and the adoption of agile principles, IN THIS DOCUMENT organizations can become more efficient. Prioritizing business requirements and driving out unnecessary scope and feature bloat are key to the new-normal efficiency. 1 Application Management and Resilience: With multiple levels of proactive self-healing mechanisms, today’s organizations are Development - making zero-downtime systems a reality. To adapt, organizations must replace or modernize their Takeaways from IBM Services applications. This means an ambidextrous approach to application modernization is needed where the organization pursues multiple different performance zones simultaneously: Research From 3 • Optimize the core: By continuing to use traditional ADM techniques, but refining them with Forrester: The Forrester Wave™: modern tools and approaches like AI, Machine Learning and Automation, organizations can Applications optimize their existing IT cores to help cut costs and maximize efficiency. -

The Frenchman Steadying Wipro's Ship
MONDAY,5JULY2021 10 NEWDELHI LONG STORY LIVEMINT.COM THEREPORTAGFRENCHMANE|TALKINGPOINTS |IDEAS |INSIGHT |THE BOTTOMLINE STEADYING WIPRO’S SHIP Leading from Paris, the new Wipro CEOThierryDelaporte is bringing fresh energy to the IT company. Wipro’snew chief executiveofficer,ThierryDelaporte, clearly has his task cut out forhim. Wipro’sITservices revenuesare still in the $8 billion rangewhile its closest competitor HCL TechnologiesLtd crossed the $10billion mark in calendar year 2020. HT Ayushman Baruah visit the Bengaluru headquarters of the role in ensuringthat the deal is asuccess. mint SECOND WIND consultingfirmEY-India.“Theyrequirea [email protected] companythathewasleading.Tomorrow, “Theteamunderherissettlingdownwell,” SHORT differentriskappetite,investmentability, BENGALURU itwouldhavebeenayearsincehetookthe he said. STORY Chart1:Afterlagging behind itspeers commercialconstruct,andtheneedtolev- job, and he is yet to set foot in Bengaluru. Delaporte aims to take a“flexible and foryears, Wipro'sm-cap hassurged past erage an ecosystemofpartnerstocreate henFrenchmanThierry open approach” to Capco’s integration, HCLTechinrecentmonths. long-term value for customers,” he said. Delaporte took the LEADING FROM AFAR giventhescaleandnatureofthebusiness. WHO Marketcapitalization (intrillion) Whenaskedaboutwhatwasworkingfor CEO’s job at IndianIT oday, Delaporte has anascent yet Capcowilloperateasaseparateglobalunit, him,Delaporte highlighted afew factors Paris-based Thierry Delaporte, WiproIHCLTechnologies nfosys Tcredibleturnaroundstorytotell.Part -

The Forrester Wave™: Next-Generation Infrastructure Outsourcing, Q4 2017 the Modern Services of Infrastructure Outsourcing by Bill Martorelli October 31, 2017
FOR INFRASTRUCTURE & OPERATIONS PROFESSIONALS The Forrester Wave™: Next-Generation Infrastructure Outsourcing, Q4 2017 The Modern Services Of Infrastructure Outsourcing by Bill Martorelli October 31, 2017 Why Read This Report Key Takeaways In our 24-criteria evaluation of next-generation HCL, Wipro, Atos, IBM, Accenture, And TCS infrastructure outsourcing providers, we identified Lead The Pack the 15 most significant ones — including Forrester’s research uncovered a market in which Accenture, Atos, Capgemini, CGI, Cognizant, HCL, Wipro, Atos, IBM, Accenture, and TCS lead DXC Technology, Fujitsu, HCL, IBM, Infosys, NTT the pack. Capgemini, Cognizant, Infosys, Fujitsu, DATA, Tata Consultancy Services (TCS), Tech DXC Technology, NTT DATA, and Tech Mahindra Mahindra, Unisys, and Wipro — and researched, offer competitive options. CGI and Unisys are analyzed, and scored them. This report shows Contenders. how each provider measures up and helps I&O I&O Pros Are Looking For Help In professionals make the right choice. Infrastructure Transformation Next-generation infrastructure outsourcing remains of interest because more I&O professionals see it as a way to address their top challenges, which include being able to thrive in a hybrid world. I&O pros require next-generation infrastructure outsourcing providers to act as strategic partners and to advise them on key infrastructure-related decisions. Emphasis On Customer Experience And Satisfaction Emerge As Key Differentiators As the traditional approach to infrastructure outsourcing becomes outdated and less effective, improved customer experience will dictate which providers will lead the pack. Furthermore, vendors that can successfully blend data center and workplace services with broad geographic reach and a differentiating value proposition will position themselves to successfully deliver next- generation infrastructure outsourcing services.