New Weapons for Natural Killer Cell Arsenal Against Hematological Malignancies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fig. L COMPOSITIONS and METHODS to INHIBIT STEM CELL and PROGENITOR CELL BINDING to LYMPHOID TISSUE and for REGENERATING GERMINAL CENTERS in LYMPHATIC TISSUES
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date Χ 23 February 2012 (23.02.2012) WO 2U12/U24519ft ft A2 (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, A61K 31/00 (2006.01) CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, PCT/US201 1/048297 KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (22) International Filing Date: ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, 18 August 201 1 (18.08.201 1) NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (25) Filing Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (26) Publication Language: English ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/374,943 18 August 2010 (18.08.2010) US kind of regional protection available): ARIPO (BW, GH, 61/441,485 10 February 201 1 (10.02.201 1) US GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, 61/449,372 4 March 201 1 (04.03.201 1) US ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (72) Inventor; and EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, ΓΓ, LT, LU, (71) Applicant : DEISHER, Theresa [US/US]; 1420 Fifth LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, Avenue, Seattle, WA 98101 (US). -

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

Remarkably Similar CTLA-4 Binding Properties of Therapeutic Ipilimumab and Tremelimumab Antibodies
www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 40), pp: 67129-67139 Research Paper Remarkably similar CTLA-4 binding properties of therapeutic ipilimumab and tremelimumab antibodies Mengnan He1,2,*, Yan Chai1,*, Jianxun Qi1, Catherine W.H. Zhang3, Zhou Tong4, Yi Shi1, Jinghua Yan4, Shuguang Tan1 and George F. Gao1,2 1CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China 2University of Chinese Academy of Sciences, Beijing 100049, China 3ImmuFuCell Biotechnology Co., Ltd., Beijing 100102, China 4CAS Key Laboratory of Microbial Physiological and Metabolic Engineering, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China *These authors have contributed equally to this work Correspondence to: George F. Gao, email: [email protected] Shuguang Tan, email: [email protected] Keywords: ipilimumab, CTLA-4, complex structure, tremelimumab Received: April 04, 2017 Accepted: April 21, 2017 Published: May 19, 2017 Copyright: He et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Monoclonal antibody based immune checkpoint blockade therapies have achieved clinical successes in management of malignant tumors. As the first monoclonal antibody targeting immune checkpoint molecules entered into clinics, the molecular basis of ipilimumab-based anti-CTLA-4 blockade has not yet been fully understood. In the present study, we report the complex structure of ipilimumab and CTLA-4. The complex structure showed similar contributions from VH and VL of ipilimumab in binding to CTLA-4 front β-sheet strands. -

Where to Study Jazz 2019
STUDENT MUSIC GUIDE Where To Study Jazz 2019 JAZZ MEETS CUTTING- EDGE TECHNOLOGY 5 SUPERB SCHOOLS IN SMALLER CITIES NEW ERA AT THE NEW SCHOOL IN NYC NYO JAZZ SPOTLIGHTS YOUNG TALENT Plus: Detailed Listings for 250 Schools! OCTOBER 2018 DOWNBEAT 71 There are numerous jazz ensembles, including a big band, at the University of Central Florida in Orlando. (Photo: Tony Firriolo) Cool perspective: The musicians in NYO Jazz enjoyed the view from onstage at Carnegie Hall. TODD ROSENBERG FIND YOUR FIT FEATURES f you want to pursue a career in jazz, this about programs you might want to check out. 74 THE NEW SCHOOL Iguide is the next step in your journey. Our As you begin researching jazz studies pro- The NYC institution continues to evolve annual Student Music Guide provides essen- grams, keep in mind that the goal is to find one 102 NYO JAZZ tial information on the world of jazz education. that fits your individual needs. Be sure to visit the Youthful ambassadors for jazz At the heart of the guide are detailed listings websites of schools that interest you. We’ve com- of jazz programs at 250 schools. Our listings are piled the most recent information we could gath- 120 FIVE GEMS organized by region, including an International er at press time, but some information might have Excellent jazz programs located in small or medium-size towns section. Throughout the listings, you’ll notice changed, so contact a school representative to get that some schools’ names have a colored banner. detailed, up-to-date information on admissions, 148 HIGH-TECH ED Those schools have placed advertisements in this enrollment, scholarships and campus life. -

Project Presentation
Technology presentation | July 2021 Fc Engineering Platform For Next Generation Therapeutic Antibodies. Property of Clayton Biotechnologies, Inc. | All rights reserved www.claytonbiotech.com Clayton Biotechnologies, Inc. www.claytonbiotech.com Page 1 Clayton Biotechnologies, Inc. is a for-profit company which facilitates the commercialization of medical discoveries made by the Clayton Foundation for Research and its supporting entities. The Clayton Foundation for Research is a nonprofit medical research organization in Houston, Texas established in 1933 by Benjamin Clayton. The two-fold mission of The Foundation is to: Ø Conduct medical research for the purpose of discovering the cause, prevention and cure of diseases for the benefit of mankind. Ø Transfer the resulting medical research discoveries from the laboratory to the use of the general public by patenting and licensing such technology for development into drugs or other products. Clayton Biotechnologies, Inc. www.claytonbiotech.com Page 2 Contents. Research at leading institutions 04 Fc Engineered Aglycosylated Antibodies. 05 Targeted therapy 06 Fc engineered antibodies 08 Signal in various immune cells 10 Platform for engineering 13 Collection of Fc Domains 14 Antibodies with exquisite affinity 18 Cytotoxic Domain – Fc1004 19 Dendritic Cell Activating Domains 27 Complement Activation Domains: Fc801, 802, 805 29 RA801 – Rituximab 32 Competition 34 Summary 35 Clayton Biotechnologies, Inc. www.claytonbiotech.com Page 3 Medical Research at Leading Research Institutions. United States of America Switzerland UCI University of Geneva Medical research for the purpose of UCSD discovering the cause, prevention and cure of diseases for the benefit of mankind. UT-Southwestern SALK UT-Austin MD Anderson UTHSC UT-Medical Branch San Antonio Baylor College of Medicine Clayton Biotechnologies, Inc. -

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

Expression Tissue
The Journal of Immunology Killer Cell Ig-Like Receptor and Leukocyte Ig-Like Receptor Transgenic Mice Exhibit Tissue- and Cell-Specific Transgene Expression1 Danny Belkin,* Michaela Torkar,* Chiwen Chang,* Roland Barten,* Mauro Tolaini,† Anja Haude,* Rachel Allen,* Michael J. Wilson,* Dimitris Kioussis,† and John Trowsdale2* To generate an experimental model for exploring the function, expression pattern, and developmental regulation of human Ig-like activating and inhibitory receptors, we have generated transgenic mice using two human genomic clones: 52N12 (a 150-Kb clone encompassing the leukocyte Ig-like receptor (LILR)B1 (ILT2), LILRB4 (ILT3), and LILRA1 (LIR6) genes) and 1060P11 (a 160-Kb clone that contains ten killer cell Ig-like receptor (KIR) genes). Both the KIR and LILR families are encoded within the leukocyte receptor complex, and are involved in immune modulation. We have also produced a novel mAb to LILRA1 to facilitate expression studies. The LILR transgenes were expressed in a similar, but not identical, pattern to that observed in humans: LILRB1 was expressed in B cells, most NK cells, and a small number of T cells; LILRB4 was expressed in a B cell subset; and LILRA1 was found on a ring of cells surrounding B cell areas on spleen sections, consistent with other data showing monocyte/macrophage expression. KIR transgenic mice showed KIR2DL2 expression on a subset of NK cells and T cells, similar to the pattern seen in humans, and expression of KIR2DL4, KIR3DS1, and KIR2DL5 by splenic NK cells. These observations indicate that linked regulatory elements within the genomic clones are sufficient to allow appropriate expression of KIRs in mice, and illustrate that the presence of the natural ligands for these receptors, in the form of human MHC class I proteins, is not necessary for the expression of the KIRs observed in these mice. -

Combination Therapies with Anti-Cd38 Antibodies Kombinationstherapien Mit Anti-Cd38-Antikörpern Polythérapies Avec Des Anticorps Anti-Cd38
(19) TZZ¥__Z¥_T (11) EP 3 110 843 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/4745 (2006.01) A61K 39/395 (2006.01) 11.09.2019 Bulletin 2019/37 A61K 31/573 (2006.01) A61K 31/65 (2006.01) A61K 31/675 (2006.01) A61P 35/00 (2006.01) (2006.01) (21) Application number: 15755187.0 A61P 35/02 (22) Date of filing: 25.02.2015 (86) International application number: PCT/US2015/017420 (87) International publication number: WO 2015/130728 (03.09.2015 Gazette 2015/35) (54) COMBINATION THERAPIES WITH ANTI-CD38 ANTIBODIES KOMBINATIONSTHERAPIEN MIT ANTI-CD38-ANTIKÖRPERN POLYTHÉRAPIES AVEC DES ANTICORPS ANTI-CD38 (84) Designated Contracting States: US-A1- 2012 231 008 US-A1- 2013 109 593 AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • SATHISH GOPALAKRISHNAN ET AL: PL PT RO RS SE SI SK SM TR "Daratumumabimproves the anti-myeloma effect Designated Extension States: of newly emerging multidrug therapies", BLOOD BA ME AND LYMPHATIC CANCER: TARGETS AND THERAPY, DOVE MEDICAL PRESS LTD, GB, vol. (30) Priority: 28.02.2014 US 201461946002 P 2013, no. 3, 18 April 2013 (2013-04-18), pages 02.06.2014 US 201462006386 P 19-24, XP002735277, ISSN: 1179-9889, DOI: 10.2147/BLCTT.S29567 (43) Date of publication of application: • RICHARDSON P ET AL: "Daratumumab. 04.01.2017 Bulletin 2017/01 Anti-CD38 monoclonal antibody, treatment of multiple myeloma", DRUGS OF THE FUTURE, (73) Proprietor: Janssen Biotech, Inc. -

New Biological Therapies: Introduction to the Basis of the Risk of Infection
New biological therapies: introduction to the basis of the risk of infection Mario FERNÁNDEZ RUIZ, MD, PhD Unit of Infectious Diseases Hospital Universitario “12 de Octubre”, Madrid ESCMIDInstituto de Investigación eLibraryHospital “12 de Octubre” (i+12) © by author Transparency Declaration Over the last 24 months I have received honoraria for talks on behalf of • Astellas Pharma • Gillead Sciences • Roche • Sanofi • Qiagen Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author Paul Ehrlich (1854-1915) • “side-chain” theory (1897) • receptor-ligand concept (1900) • “magic bullet” theory • foundation for specific chemotherapy (1906) • Nobel Prize in Physiology and Medicine (1908) (together with Metchnikoff) Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author 1981: B-1 antibody (tositumomab) anti-CD20 monoclonal antibody 1997: FDA approval of rituximab for the treatment of relapsed or refractory CD20-positive NHL 2001: FDA approval of imatinib for the treatment of chronic myelogenous leukemia Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author Functional classification of targeted (biological) agents • Agents targeting soluble immune effector molecules • Agents targeting cell surface receptors -
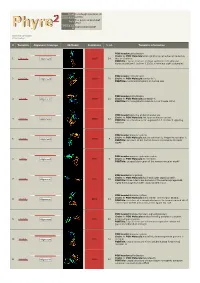
Phyre 2 Results for A1YIY0
Email [email protected] Description A1YIY0 Tue Jul 30 13:19:15 BST Date 2013 Unique Job 1035bc4b501530df ID Detailed template information # Template Alignment Coverage 3D Model Confidence % i.d. Template Information PDB header:cell adhesion Chain: A: PDB Molecule:down syndrome cell adhesion molecule 1 c3dmkA_ Alignment 100.0 14 (dscam) isoform PDBTitle: crystal structure of down syndrome cell adhesion molecule (dscam)2 isoform 1.30.30, n-terminal eight ig domains PDB header:cell adhesion 2 c2om5A_ Alignment 100.0 20 Chain: A: PDB Molecule:contactin 2; PDBTitle: n-terminal fragment of human tax1 PDB header:cell adhesion 3 c3jxaA_ Alignment 100.0 21 Chain: A: PDB Molecule:contactin 4; PDBTitle: immunoglobulin domains 1-4 of mouse cntn4 PDB header:signaling protein/transferase Chain: A: PDB Molecule:tek tyrosine kinase variant; 4 c4k0vA_ 100.0 12 Alignment PDBTitle: structural basis for angiopoietin-1 mediated signaling initiation PDB header:immune system Chain: A: PDB Molecule:natural cytotoxicity triggering receptor 1; 5 c1p6fA_ 100.0 9 Alignment PDBTitle: structure of the human natural cytotoxicity receptor nkp46 PDB header:immune system/receptor 6 c1ollA_ Alignment 99.9 9 Chain: A: PDB Molecule:nk receptor; PDBTitle: extracellular region of the human receptor nkp46 PDB header:viral protein Chain: A: PDB Molecule:hoc head outer capsid protein; 7 c3shsA_ 99.9 18 Alignment PDBTitle: three n-terminal domains of the bacteriophage rb49 highly immunogenic2 outer capsid protein (hoc) PDB header:immune system Chain: E: PDB Molecule:natural -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Flow Reagents Single Color Antibodies CD Chart
CD CHART CD N° Alternative Name CD N° Alternative Name CD N° Alternative Name Beckman Coulter Clone Beckman Coulter Clone Beckman Coulter Clone T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells CD1a T6, R4, HTA1 Act p n n p n n S l CD99 MIC2 gene product, E2 p p p CD223 LAG-3 (Lymphocyte activation gene 3) Act n Act p n CD1b R1 Act p n n p n n S CD99R restricted CD99 p p CD224 GGT (γ-glutamyl transferase) p p p p p p CD1c R7, M241 Act S n n p n n S l CD100 SEMA4D (semaphorin 4D) p Low p p p n n CD225 Leu13, interferon induced transmembrane protein 1 (IFITM1). p p p p p CD1d R3 Act S n n Low n n S Intest CD101 V7, P126 Act n p n p n n p CD226 DNAM-1, PTA-1 Act n Act Act Act n p n CD1e R2 n n n n S CD102 ICAM-2 (intercellular adhesion molecule-2) p p n p Folli p CD227 MUC1, mucin 1, episialin, PUM, PEM, EMA, DF3, H23 Act p CD2 T11; Tp50; sheep red blood cell (SRBC) receptor; LFA-2 p S n p n n l CD103 HML-1 (human mucosal lymphocytes antigen 1), integrin aE chain S n n n n n n n l CD228 Melanotransferrin (MT), p97 p p CD3 T3, CD3 complex p n n n n n n n n n l CD104 integrin b4 chain; TSP-1180 n n n n n n n p p CD229 Ly9, T-lymphocyte surface antigen p p n p n