Study of Physico-Chemical and Micronutrient Status of Soil, Water
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sargodha District Sargodha
DISTRICT DISASTER MANAGEMENT PLAN 2020 DIVISION SARGODHA DISTRICT SARGODHA IDP Camp in 2009 Earthquake Flood Mock Exercise in May 2020 Corona virus Pandemic-Training to wear PPE’s Prepared by: MAZHAR SHAH, DISTRICT EMERGENCY OFFICER Approved by: DDMA Sargodha DDMP 2020 TABLE OF CONTENTS Executive Summary .................................................................................................................................................... 1 Aim and Objectives ..................................................................................................................................................... 2 District Profile .............................................................................................................................................................. 3 Coordination Mechanism ............................................................................................................................................ 9 Risk Analysis............................................................................................................................................................ 19 Mitigation Strategy ................................................................................................................................................... 25 Early Warning .......................................................................................................................................................... 28 Rescue Strategy ..................................................................................................................................................... -

Ethnomedicinal Recipes of Wild Fruits of Sargodha District, Punjab, Pakistan
J. Bio. & Env. Sci. 2019 Journal of Biodiversity and Environmental Sciences (JBES) ISSN: 2220-6663 (Print) 2222-3045 (Online) Vol. 14, No. 6, p. 262-271, 2019 http://www.innspub.net RESEARCH PAPER OPEN ACCESS Ethnomedicinal recipes of wild fruits of Sargodha District, Punjab, Pakistan Asif Abbas Shah1, Amin Shah1, Muhammad Nadeem2, Sarvat Rahim*1 1Department of Botany, University of Sargodha, Sargodha, Pakistan 2Institute of Food Science and Nutrition, University of Sargodha, Sargodha, Pakistan Article published on June 30, 2019 Key words: Wild fruit, Indigenous communities, Folklore, Curative flora. Abstract The The present study was conducted in the Sargodha district, Punjab, to underline indigenous knowledge of wild edible fruits as for therapeutic use by the local inhabitants. Folklore traditions with respect to curative flora were gathered through meetings and surveys from key informants including local residents, shepherds, herders, ranchers, traditional healers and herbalists. Rural vicinities were engaged to investigate reliable clients of wild edible fruits. Such studies not just assume a significant role in saving the native medica-ethnobotanical information yet in addition help to update the social status and economic values of the indigenous communities that consequently preserve the global heritage. 27 fruit species belonging to 15 families were recorded in the territory. All the plants were extremely compelling against various diseases and native to the area. The data about their botanical names, local names, families, part used, method of use and used for, all were recorded. The fruits and other plant samples of the investigated plants were collected, identified, preserved and deposited in the Herbarium of University of Sargodha (SARGU) for future references. -

Audit Report on the Accounts of Tehsil Municipal Administrations District Sargodha
AUDIT REPORT ON THE ACCOUNTS OF TEHSIL MUNICIPAL ADMINISTRATIONS DISTRICT SARGODHA AUDIT YEAR 2015-16 AUDITOR GENERAL OF PAKISTAN TABLE OF CONTENTS ABBREVIATIONS & ACRONYMS ..................................................... i PREFACE .............................................................................................. ii EXECUTIVE SUMMARY ................................................................... iii SUMMARY TABLES AND CHARTS................................................ vii Table 1: Audit Work Statistics ............................................................... vii Table 2: Audit Observations regarding Financial Management ............... vii Table 3: Outcome Statistics ................................................................... viii Table 4: Irregularities Pointed Out ........................................................ viii Table 5: Cost-Benefit ............................................................................ viii CHAPTER-1 .......................................................................................... 1 1.1 TEHSIL MUNICIPAL ADMINISTRATIONS, DISTRICT SARGODHA .......................................................................................... 1 1.1.1 Introduction ................................................................................. 1 1.1.2 Comments on Budget and Accounts (Variance Analysis).............. 2 1.1.3 Brief Comments on the Status of Compliance on MFDAC Paras of Audit Year 2014-15 ..................................................................... -

Water Supply & Sanitation
WATER SUPPLY & SANITATION VISION Provision of adequate, safe drinking water and sanitation facilities to the entire rural and urban communities of Punjab through equitable, efficient and sustainable services. WATER & SANITATION POLICY Drinking Water Policy: Safe drinking water is accessible at premises, available when needed and free from contamination on sustainable basis to whole population of Punjab in addition to acquiring and adopting improved knowledge about safe drinking water, sanitation and hygiene in their daily life. Sanitation Policy: Policy envisions developing a safely managed sanitation environment for all citizens of the province, contributing towards high quality life in Punjab. OBJECTIVES . To achieve Sustainable Development Goals and its targets ( Goal 6.1 & 6.2 of SDGs) . Improving standards of public health through provision of improved services backed by legal, regulatory and binding framework. Laying down a roadmap for mobilization of resources to ensure provision of drinking water & sanitation to all by targeted timelines, assigning a priority to unserved and under-served areas . Focusing on capacity building of local governments and promoting Public- Private Partnerships to improve the operation and maintenance of water supply & sanitation schemes . To raise living standard of communities by providing quality drinking water and improved sanitation services . To reduce the spread of water borne diseases 123 ECONOMIC IMPACT OF WATER AND SANITATION PROJECTS The need for improved water and sanitation infrastructure is fundamental to the wellbeing of all citizens and increased coverage of these essential services will significantly contribute to socio-economic development. A study conducted by World Bank (2012) for Pakistan has shown that impact of poor sanitation and hygiene has cost the economy PKR 344 billion (US$ 6.0 billion) annually in 2006, or the equivalent to 3.9% of Gross Domestic Product (GDP). -

Coversheet for Thesis in Sussex Research Online
A University of Sussex DPhil thesis Available online via Sussex Research Online: http://sro.sussex.ac.uk/ This thesis is protected by copyright which belongs to the author. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the Author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the Author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Please visit Sussex Research Online for more information and further details PATRONS, BROTHERS AND LANDLORDS: COMPETING FOR THE VOTE IN RURAL PAKISTAN Shandana Khan Mohmand Doctor of Philosophy Institute of Development Studies University of Sussex May 2011 ii I hereby declare that this thesis has not been and will not be, submitted in whole or in part to another University for the award of any other degree. Signature iii University of Sussex Shandana Khan Mohmand Doctor of Philosophy Patrons, Brothers and Landlords: Competing for the Vote in Rural Pakistan SUMMARY How do citizens vote in rural Pakistan, and how much agency do they have in relation to local landlords, patrons and kinship networks in making electoral decisions? I explore this question in this dissertation through an empirical investigation of the voting behaviour of Pakistan’s rural majority in its most populous and politically important province, Punjab, using a mix of qualitative and quantitative methods and original data on the voting behaviour of about 2300 households in 38 villages. -

Seroprevalence and Spatial Distribution of Toxoplasmosis in Sheep and Goats in North-Eastern Region of Pakistan
ISSN (Print) 0023-4001 ISSN (Online) 1738-0006 Korean J Parasitol Vol. 54, No. 4: 439-446, August 2016 ▣ ORIGINAL ARTICLE http://dx.doi.org/10.3347/kjp.2016.54.4.439 Seroprevalence and Spatial Distribution of Toxoplasmosis in Sheep and Goats in North-Eastern Region of Pakistan Haroon Ahmed1,2, Ayesha Malik3, Muhammad Arshad3, Irfan Mustafa4, Mobushir Riaz Khan5, 6 7 8 2, Muhammad Sohail Afzal ,Shahzad Ali , Muhammad Mobeen , Sami Simsek * 1Department of Biosciences,COMSATS Institute of Information Technology (CIIT), Islamabad, Pakistan; 2Department of Parasitology, Faculty of Veterinary, University of Firat, 23119, Elazig, Turkey; 3Department of Zoology, University of Sargodha, Pakistan; 4 Department of Biology, Government Postgraduate College Chishtian, Punjab, Pakistan; 5Department of Remote Sensing & GIS, PMAS-University of Arid Agriculture, Rawalpindi, Pakistan; 6Department of Chemistry, School of Science, UMT Lahore, Pakistan; 7Department of Wildlife and Ecology, University of Veterinary and Animal Sciences, Lahore, Pakistan; 8Department of Earth Sciences, University of Sargodha, Pakistan Abstract: Toxoplasmosis is a protozoan disease that is caused by Toxoplasma gondii in livestock and humans. Due to its medical and veterinary importance, it is essential to study the seroprevalence of T. gondii infection among humans and animals in various parts of the world. The major objective of this study was to determine the seroprevalence and spatial distribution of toxoplasmosis in small ruminants (sheep and goats) of north-eastern region, Pakistan. A total of 1,000 ani- mals comprising of sheep (n= 470) and goats (n= 530) were examined for T. gondii infection by using ELISA. An epidemio- logical data was collected in the form of questionnaire. -
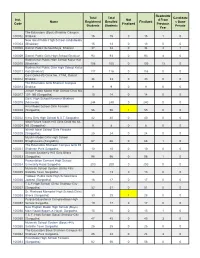
Inst. Code Name Total Registered Students Total Enrolled Students
Readmitte Total Total Candidate Inst. Not d From Name Registered Enrolled Finalized s Gone Code Finalized Previous Students Students Private Year The Educators (Boys) Bhakkar Campus 100002 Bhakkar 15 15 0 15 1 0 New Ideal Public High School Jandanwala 100003 (Bhakkar) 15 13 0 13 0 0 100008 District Public School Boys, Bhakkar 37 34 0 34 3 1 100009 District Public Girls High School Bhakkar 93 93 0 93 4 0 Roshan Kal Public High School Kallur Kot 100010 (Bhakkar) 108 105 0 105 13 0 Roshan Kal Public Girls High School Kallur 100011 Kot (Bhakkar) 117 116 0 116 0 0 Govt Girls H/S Chak No. 71/ML District 100012 Bhakkar. 34 33 0 33 0 0 The Educators Girls Bhakkar Campus 100014 Bhakkar 9 9 0 9 0 0 Jinnah Public Model High School Chak No 100017 107- NB (Sargodha) 15 14 0 14 0 0 Govt. High School Kammar Mashani 100019 (Mianwali) 244 240 0 240 0 0 Hira Model School Girls Farooka 100020 (Sargodha) 56 56 1 55 0 0 100022 Kims Girls High School N.S.T Sargodha. 42 40 0 40 0 0 Ideal Future Vision H/S Girls Chak No 84- 100024 NB (Sargodha) 9 8 0 8 0 0 Islamic Ideal School Girls Farooka 100029 (Sargodha). 25 24 0 24 0 0 Muslim Model Girls High School 100030 Bhagtanwala (Sargodha) 67 66 0 66 1 0 The Educators Shaheen Campus Girls 93 100031 Shaheen Park Sargodha. 10 10 0 10 0 0 Science Academy H/S Girls Bhera 100033 (Sargodha). -

Grand Total Bhalwal Bhera Kot Momin Sahiwal
TEHSIL WISE SUMMARY OF RATIONALIZATION DISTRICT SARGODHA Tehsil DEO (M) DEO (SE) DEO (W) Grand Total Bhalwal 1 17 12 30 Bhera 1 5 4 10 Kot Momin 9 8 17 Sahiwal 4 10 6 20 Sargodha 14 42 13 69 Shahpur 5 7 6 18 Sillanwali 1 13 3 17 Grand Total 35 102 44 181 Sr. # NAME CONTACT # Wing Tehsil FROM SCHOOL TO SCHOOL DESIGNATION DEO (W) Bhera 38470732 - GGES KHALID ENGLISH MEDIUM BHERA 38470725 - GGPS MC NO.2 BHERA PST (Arts) - 14 3074863281 (غزالہ یاسمین) Ghazala Yasmeen 1 DEO (SE) Sargodha 38420662 - GGHS MC BLOCK NO.14 SARGODHA 38420631 - GGES MUHAMMAD ABAD (MUHAMMADI COLONY) SGD PST (Arts) - 14 3037687251 (فہمیدہ بیگم) Fehmeeda Begum 2 DEO (W) Bhalwal 38410531 - GGES CHAK NO. 7 SB SYEDAN WALA 38410528 - GMPS CHAK NO.9 NB LOKRI PST (Arts) - 14 3043700779 (نرگس سلطانہ) Nargis Sultana 3 DEO (W) Bhalwal 38410522 - GGES CHAK NO.14 NB 38410510 - GGES CHAK NO.11 ML PST (Science/Maths) - 14 3046068081 (انمول حنا) Anmol Hina 4 DEO (SE) Sargodha 38420427 - GHS CHAK NO.52 A-NB 38420403 - GPS MC BLOCK NO.23-A SARGODHA PST - 14 3458627649 (هللا دتہ) Allah Ditta 5 DEO (SE) Sargodha 38420385 - GHS MITHA LAK 38420305 - GPS CHAK NO.76 SB PST (Arts) - 14 3450756592 (چوہدری دمحم الطاف) M Altaf 6 DEO (W) Shahpur 38430409 - GGES NO.1 JHAWARIAN 38430395 - GGPS BHABHRANI PST (Arts) - 14 3041045232 (زاہدہ پروین) Zahida Perveen 7 DEO (SE) Shahpur 38430016 - GHS JALPANA SHAHPUR 38420440 - GPS CHAK NO.4 RAKH DHAREMA PST (Arts) - 14 3018899609 (دمحم نواز) Muhammad Nawaz 8 9 Muhammad Sarfraz 3336790967 DEO (M) Shahpur 38430168 - GPS HUKAM PUR 38430196 - GPS SULTAN PUR -

Water Supply & Sanitation
WATER SUPPLY & SANITATION VISION Provision of adequate, safe drinking water and sanitation facilities to the entire rural and urban communities of Punjab through equitable, efficient and sustainable services. WATER & SANITATION POLICY . Government of the Punjab aims to provide safe drinking water of an adequate quantity at an affordable cost through equitable, efficient and sustainable services to all citizens (Drinking Water Policy Approved in May, 2011). The goal of Provincial sanitation policy is to ensure that entire population of Punjab has access to safe and affordable sanitation for a quality life. (Punjab Sanitation Policy has been formulated recognizing the significance of sanitation which is under approval). OBJECTIVES . Improving standards of public health through provision of improved services supported up by legal, regulatory and binding framework. Laying down a roadmap for mobilization of resources to ensure provision of drinking water & sanitation to all by the target timeline, assigning a priority to un- served and under-served areas. Focusing on capacity building of local governments and Public-Private Partnerships to improve the operation and maintenance of water supply & sanitation schemes. Facilitating the introduction and institutionalization of an effective Monitoring and Evaluation System, this includes performance benchmark in service delivery. To raise living standards of community by providing quality drinking water and improved sanitation services. To reduce the spread of water borne diseases. To achieve Sustainable Development Goals and targets. 205 ECONOMIC IMPACT OF WATER AND SANITATION PROJECTS The need for water and sanitation is fundamental to all citizens, and increased coverage of these essential services will significantly contribute to population welfare as well as wealth and stability of citizens. -
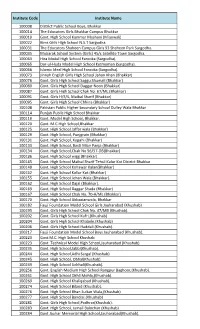
Institute Code Institute Name
Institute Code Institute Name 100008 District Public School Boys, Bhakkar 100014 The Educators Girls Bhakkar Campus Bhakkar 100019 Govt. High School Kammar Mashani (Mianwali) 100022 Kims Girls High School N.S.T Sargodha. 100031 The Educators Shaheen Campus Girls 93 Shaheen Park Sargodha. 100035 Mubarak School System (Girls) 45/c Satellite Town Sargodha. 100063 Hira Model High School Farooka (Sargodha). 100065 Dar-ul-Huda Model High School Kotmoman (Sargodha). 100066 Islamic Ideal High School Farooka (Sargodha). 100073 Jinnah English Girls High School Jahan Khan (Bhakkar) 100076 Govt. Girls High School Saggu Shumali (Bhakkar) 100080 Govt. Girls High School Daggar Noon (Bhakkar) 100087 Govt. Girls High School Chak No. 67/ML (Bhakkar) 100091 Govt. Girls H/S/S, Maibal Sharif (Bhakkar) 100095 Govt. Girls High School Chhina (Bhakkar) 100108 Pakistan Public Higher Secondary School Dulley Wala Bhakkar 100114 Punjab Public High School Bhakkar 100119 Govt. Model High School, Bhakkar. 100120 Govt. M.C.High School,Bhakkar 100125 Govt. High School Jaffar wala (Bhakkar) 100129 Govt. High School, Punjgrain (Bhakkar) 100131 Govt. High School, Kapahi (Bhakkar) 100133 Govt. High School, Basti Mian Panja (Bhakkar) 100134 Govt. High School,Chak No 56/57-DB(Bhakkar) 100136 Govt. High School wigg (Bhakkar) 100145 Govt. High School Maibal Sharif Tehsil Kalur Kot District Bhakkar 100148 Govt. High School Kohawar Kalan(Bhakkar) 100152 Govt. High School Kallur Kot (Bhakkar) 100155 Govt. High School Jehan Wala (Bhakkar). 100162 Govt. High School Dajal (Bhakkar). 100163 Govt. High School Daggar Shada (Bhakkar) 100167 Govt. High School Chak No. 70-A/ML (Bhakkar) 100170 Govt. High School Abbasianwala, Bhakkar 100182 Fauji Foundation Model School Girls Jauharabad (Khushab) 100195 Govt. -

Audit Report on the Accounts of District Government Sargodha Audit Year
AUDIT REPORT ON THE ACCOUNTS OF DISTRICT GOVERNMENT SARGODHA AUDIT YEAR 2016-17 AUDITOR GENERAL OF PAKISTAN TABLE OF CONTENTS ABBREVIATIONS & ACRONYMS ....................................................... i PREFACE .................................................................................................. ii EXECUTIVE SUMMARY ..................................................................... iii SUMMARY TABLES AND CHARTS ................................................. vii Table 1: Audit Work Statistics .................................................... vii Table 2: Audit observation regarding Financial Management .... vii Table 3: Outcome Statistics ........................................................ vii Table 4: Irregularities Pointed Out ............................................. viii Table 5: Cost-Benefit ................................................................. viii CHAPTER-1 .............................................................................................. 1 1.1 District Government, Sargodha ............................................... 1 1.1.1 Introduction of Departments ................................................... 1 1.1.2 Comments on Budget and Accounts (Variance Analysis) ...... 1 1.1.3 Brief Comments on the Status of MFDAC Audit Paras of Audit Report 2015-16.............................................................. 3 1.1.4 Brief Comments on the Status of Compliance with PAC Directives ................................................................................ 3 1.2 -

Sargodha Blockwise
POPULATION AND HOUSEHOLD DETAIL FROM BLOCK TO DISTRICT LEVEL PUNJAB (SARGODHA DISTRICT) ADMIN UNIT POPULATION NO OF HH SARGODHA DISTRICT 3,703,588 600,224 BHALWAL TEHSIL 357,331 64,441 BHALWAL MC 100,135 18349 CHARGE NO 05 100,135 18349 CIRCLE NO 01 14,077 2605 135050101 2,457 446 135050102 1,191 208 135050103 1,830 355 135050104 1,227 208 135050105 1,556 260 135050106 2,164 373 135050107 820 199 135050108 1,201 210 135050109 1,631 346 CIRCLE NO 02 14,215 2749 135050201 1,548 285 135050202 891 157 135050203 1,523 268 135050204 1,978 423 135050205 562 90 135050206 960 199 135050207 1,559 266 135050208 2,586 516 135050209 666 100 135050210 1,654 384 135050211 288 61 CIRCLE NO 03 14,927 2538 135050301 2,052 404 135050302 2,142 330 135050303 849 123 135050304 1,274 213 135050305 1,356 184 135050306 755 101 135050307 1,116 225 135050308 1,240 205 135050309 1,618 309 135050310 722 144 135050311 1,803 300 CIRCLE NO 04 17,617 3005 135050401 1,848 254 135050402 2,364 407 135050403 2,332 384 135050404 1,081 201 135050405 2,139 388 135050406 1,861 330 135050407 3,721 596 135050408 2,271 445 CIRCLE NO 05 15,206 2912 135050501 2,281 439 135050502 1,154 254 Page 1 of 98 POPULATION AND HOUSEHOLD DETAIL FROM BLOCK TO DISTRICT LEVEL PUNJAB (SARGODHA DISTRICT) ADMIN UNIT POPULATION NO OF HH 135050503 962 153 135050504 2,482 495 135050505 1,763 274 135050506 2,897 554 135050507 1,273 213 135050508 1,273 288 135050509 1,121 242 CIRCLE NO 06 10,515 2049 135050601 854 185 135050602 952 197 135050603 817 108 135050604 688 130 135050605 1,805 388 135050606 2,366