Pituitrin-Injection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Ins and Outs of Circadian Timekeeping Steven a Brown* and Ueli Schibler†
gd9507.qxd 11/10/1999 12:14 PM Page 588 588 The ins and outs of circadian timekeeping Steven A Brown* and Ueli Schibler† Recent research in Drosophila and in mammals has generated The mechanism by which light signals entrain the clock is fascinating new models for how circadian clocks in these another topic of intense interest, and will be our other focus. organisms are reset by light and how these clocks, in turn, direct circadian outputs. Though light perception by the central clock is Central clock mechanisms: a brief summary ocular in mammals, it probably proceeds via a mechanism In all cases examined to date, circadian clocks have been separate from traditional visual transduction. In Drosophila, one cell-autonomous: a single cell can generate and maintain mechanism is non-ocular and is in fact present in many different self-sustained circadian oscillations. The molecular basis tissues. In both organisms, the cryptochrome family of for these rhythms may rely on a negative feedback loop in photoreceptor-like molecules plays a role in the circadian clock, which clock proteins negatively regulate their own abun- though their function is incompletely understood. Moreover, dance or activity. This regulation may occur both at the although a master clock resides in the brain, a functional clock transcriptional and at the post-transcriptional level. For appears to reside in most cells of the body. In these tissues, at example, in the bread mold Neurospora crassa, the Fre- least some output genes are controlled at the transcriptional quency protein negatively regulates its own transcription level directly by clock proteins; others appear to be regulated by by interfering with the ability of the White-collar-1 and cascades of circadian transcription factors. -

The Islet Ghrelin Cell 52:1 R35–R49 Review
N WIERUP and others The islet ghrelin cell 52:1 R35–R49 Review The islet ghrelin cell Nils Wierup, Frank Sundler and R Scott Heller1 Correspondence should be addressed Unit of Neuroendocrine Cell Biology, Department of Clinical Sciences in Malmo¨ , Lund University Diabetes Centre, to N Wierup Clinical Research Centre, Scania University Hospital, Jan Waldenstro¨ ms gata 35, SE 205 02 Malmo¨ , Sweden Email 1Imaging Team, Novo Nordisk A/S, Novo Nordisk Park, DK2760 Ma˚ løv, Denmark [email protected] Abstract The islets of Langerhans are key regulators of glucose homeostasis and have been known Key Words as a structure for almost one and a half centuries. During the twentieth century several " ghrelin different cell types were described in the islets of different species and at different " islet developmental stages. Six cell types with identified hormonal product have been described " ghrelin cell so far by the use of histochemical staining methods, transmission electron microscopy, " pancreas and immunohistochemistry. Thus, glucagon-producing a-cells, insulin-producing b-cells, " human somatostatin-producing d-cells, pancreatic polypeptide-producing PP-cells, serotonin- " rat producing enterochromaffin-cells, and gastrin-producing G-cells have all been found in the " mouse mammalian pancreas at least at some developmental stage. Species differences are at hand " diabetes and age-related differences are also to be considered. Eleven years ago a novel cell type, " development the ghrelin cell, was discovered in the human islets. Subsequent studies have shown the presence of islet ghrelin cells in several animals, including mouse, rat, gerbils, and fish. The developmental regulation of ghrelin cells in the islets of mice has gained a lot of interest and several studies have added important pieces to the puzzle of molecular mechanisms and the genetic regulation that lead to differentiation into mature ghrelin cells. -

Electron Microscopy and Histochemical Correlation of Human Anterior Pituitary Cells
Electron Microscopy and Histochemical Correlation of Human Anterior Pituitary Cells Carlos Paiz, MD and Gordon R. Hennigar, MD THE PARS ANrERIOR of human adenohypophysis has been studied extensively by histochemical and tinctorial methods."-8 Multiple nomenclatures have been developed to describe the various cell types, but the ultimate goal is to adopt a terminology based solely on function. Electron microscopy has served to clarify in part the fine structure of the adenohypophysis, and its contribution to date has been the attempted correlation of granular size and shape with specific hormonal secretion.9-" Similar studies have been made in animal species other than man.12-'7 In several human cell types, the granules are so similar that it is fre- quently difficult if not impossible to distinguish the cells on morphologic grounds alone. The use of thick-thin section correlation for light and electron microscopy can, in part, clarify this problem, since histochem- ical properties at the light level may be correlated with fine structural differences within the same cell at the electron microscopic level. Such correlations have served three purposes: (1) We have been able to relate certain serous, mucoid, and seemingly chromophobe cells of light microscopy with the corresponding electron microscopic equiv- alents. (2) In so doing, we have demonstrated that cells having the same or similar granule morphology with electron microscopy are strik- ingly different with light microscopy histochemistry. (3) The thick-thin section method of comparison has provided us with the opportunity to relate fine structural differences within pituitary cells with the corre- sponding variable dye binding seen in a single cell with light micros- copy. -

Regulating Distinct Cell Lineages in the Pancreatic Islet Joshua a Levine
Regulating Distinct Cell Lineages in the Pancreatic Islet Joshua A Levine Submitted in partial fulfillment of the Requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2013 © 2012 Joshua A Levine All Rights Reserved ABSTRACT Regulating Distinct Cell Lineages in the Pancreatic Islet Joshua A Levine Type I and type II diabetes mellitus are associated with a loss of functioning insulin- producing β cells in the pancreas. Understanding the mechanism of normal islet and β cell development will be an important step in developing possible treatments for the disease. Nkx2.2 is essential for proper β cell differentiation. Nkx2.2-/- mice show a complete absence of insulin-producing β cells, a 90% reduction of glucagon-producing α cells, and an increase in ghrelin-producing cells. Nkx2.2 contains three conserved domains: the tinman domain (TN), homeodomain (HD), and NK2-specific domain (SD). The SD domain is highly conserved among Nk2 family members and across species. However, its function remains largely unknown. In order to further understand the molecular interactions involving Nkx2.2 in the developing mouse pancreas, we have generated a mouse line containing mutations in the NK2-SD domain. We show that SD mutant mice have a decrease in β cell numbers as well as a decrease in the β cell markers, NeuroD, Nkx6.1, Ins1 and Ins2. However, there is no change in α cell numbers or the α cell markers, Glucagon and Irx2. Unlike the persistent upregulation of ghrelin in the Nkx2.2-/- mice, Nkx2.2SD/SD mice display a transient increase in ghrelin expression, which normalizes by birth. -

Embryonic Endocrine Pancreas and Mature Β Cells Acquire Α and PP Cell Phenotypes Upon Arx Misexpression
Embryonic endocrine pancreas and mature β cells acquire α and PP cell phenotypes upon Arx misexpression Patrick Collombat, … , Palle Serup, Ahmed Mansouri J Clin Invest. 2007;117(4):961-970. https://doi.org/10.1172/JCI29115. Research Article Aristaless-related homeobox (Arx) was recently demonstrated to be involved in pancreatic α cell fate specification while simultaneously repressing the β and δ cell lineages. To establish whether Arx is not only necessary, but also sufficient to instruct the α cell fate in endocrine progenitors, we used a gain-of-function approach to generate mice conditionally misexpressing this factor. Mice with forced Arx expression in the embryonic pancreas or in developing islet cells developed a dramatic hyperglycemia and eventually died. Further analysis demonstrated a drastic loss of β and δ cells. Concurrently, a remarkable increase in the number of cells displaying α cell or, strikingly, pancreatic polypeptide (PP) cell features was observed. Notably, the ectopic expression of Arx induced in embryonic or adult β cells led to a loss of the β cell phenotype and a concomitant increase in a number of cells with α or PP cell characteristics. Combining quantitative real-time PCR and lineage-tracing experiments, we demonstrate that, in adult mice, the misexpression of Arx, rather than its overexpression, promotes a conversion of β cells into glucagon- or PP-producing cells in vivo. These results provide important insights into the complex mechanisms underlying proper pancreatic endocrine cell allocation and cell identity acquisition. Find the latest version: https://jci.me/29115/pdf Related Commentary, page 859 Research article Embryonic endocrine pancreas and mature β cells acquire α and PP cell phenotypes upon Arx misexpression Patrick Collombat,1 Jacob Hecksher-Sørensen,2 Jens Krull,1 Joachim Berger,3 Dietmar Riedel,4 Pedro L. -

Renal Cell Carcinoma: from a Pathologist's Perspective
SMGr up Histologic Aspect of Renal Cell Carcinomas Solène-Florence Kammerer-Jacquet and Nathalie Rioux-Leclercq* Department*Corresponding of Pathology, author: Pontchaillou Hospital, France Nathalie Rioux-Leclercq, Department of Pathology, Pontchaillou Hospital, 2 rue Henri le Guilloux, 35300 Rennes Cedex 9, France, Tel: +33 2 99 28 42 79; Fax: +Published 33 2 99 28 Date: 42 84; Email: [email protected] July 18, 2016 ABSTRACT the ISUP (International Society of Urologic Pathology). The most recent recommendations were International guidelines for the classification of renal tumors in adults are provided from (RCC). In this established in 2012, and the 2016 WHO classification incorporated these guidelines but also clinical, pathological, and molecular characteristics of the renal cell carcinomas review, we focus on the macroscopic, histologic immunohistochemical and cytogenetic criteria (ccRCC) (P-RCC) (Ch-RCC), MiT family translocation RCC, that lead to the diagnosis of RCC. The main histologic subtypes of RCC include clear cell RCC collecting duct carcinoma, and medullary renal cell carcinoma. We also describe the other and rare , papillary RCC , chromophobe RCC entities of RCC recognized in the 2016 WHO classification: hereditary leiomyomatosis associated RCC, succinate dehydrogenase deficient RCC, mucinous tubular and spindle cell carcinoma, (AML). tubulocystic RCC, acquired cystic disease associated RCC, mixed epithelial and stromal tumor of Renalthe kidney, Cell Carcinoma clear cell | www.smgebooks.com papillary RCC, and epithelioid angiomyolipoma 1 Copyright Rioux-Leclercq N.This book chapter is open access distributed under the Creative Commons Attribu- tion 4.0 International License, which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited. -

Regulation of Neurod1 Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas
Regulation of Neurod1 Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas Teresa L. Mastracci1¤a, Keith R. Anderson2.¤b, James B. Papizan1., Lori Sussel1,2* 1 Department of Genetics and Development, Russ Berrie Medical Pavilion, Columbia University, New York, New York, United States of America, 2 Molecular Biology Program, University of Colorado Denver Health Sciences Center, Aurora, Colorado, United States of America Abstract During pancreatic development, transcription factor cascades gradually commit precursor populations to the different endocrine cell fate pathways. Although mutational analyses have defined the functions of many individual pancreatic transcription factors, the integrative transcription factor networks required to regulate lineage specification, as well as their sites of action, are poorly understood. In this study, we investigated where and how the transcription factors Nkx2.2 and Neurod1 genetically interact to differentially regulate endocrine cell specification. In an Nkx2.2 null background, we conditionally deleted Neurod1 in the Pdx1+ pancreatic progenitor cells, the Neurog3+ endocrine progenitor cells, or the glucagon+ alpha cells. These studies determined that, in the absence of Nkx2.2 activity, removal of Neurod1 from the Pdx1+ or Neurog3+ progenitor populations is sufficient to reestablish the specification of the PP and epsilon cell lineages. Alternatively, in the absence of Nkx2.2, removal of Neurod1 from the Pdx1+ pancreatic progenitor population, but not the Neurog3+ endocrine progenitor cells, restores alpha cell specification. Subsequent in vitro reporter assays demonstrated that Nkx2.2 represses Neurod1 in alpha cells. Based on these findings, we conclude that, although Nkx2.2 and Neurod1 are both necessary to promote beta cell differentiation, Nkx2.2 must repress Neurod1 in a Pdx1+ pancreatic progenitor population to appropriately commit a subset of Neurog3+ endocrine progenitor cells to the alpha cell lineage. -

Molecular Genetics and Immunohistochemistry Characterization of Uncommon and Recently Described Renal Cell Carcinomas
Review Article Molecular genetics and immunohistochemistry characterization of uncommon and recently described renal cell carcinomas Qiu Rao1*, Qiu-Yuan Xia1*, Liang Cheng2, Xiao-Jun Zhou1 1Department of Pathology, Jinling Hospital, Nanjing University School of Medicine, Nanjing, China; 2Department of Pathology and Laboratory, Indiana University School of Medicine, Indianapolis, IN, USA *These authors contributed equally to this work. Correspondence to: Dr. Xiao-Jun Zhou. Department of Pathology, Nanjing Jinling Hospital, Nanjing University School of Medicine, Nanjing, Jiangsu 210002, China. Email: [email protected]. Abstract: Renal cell carcinoma (RCC) compromises multiple types and has been emerging dramatically over the recent several decades. Advances and consensus have been achieved targeting common RCCs, such as clear cell carcinoma, papillary RCC and chromophobe RCC. Nevertheless, little is known on the characteristics of several newly-identified RCCs, including clear cell (tubulo) papillary RCC, Xp11 translocation RCC, t(6;11) RCC, succinate dehydrogenase (SDH)-deficient RCC, acquired cystic disease- associated RCC, hereditary leiomyomatosis RCC syndrome-associated RCC, ALK translocation RCC, thyroid-like follicular RCC, tubulocystic RCC and hybrid oncocytic/chromophobe tumors (HOCT). In current review, we will collect available literature of these newly-described RCCs, analyze their clinical pathologic characteristics, discuss their morphologic and immunohistologic features, and finally summarize their molecular and genetic evidences. We expect this review would be beneficial for the understanding of RCCs, and eventually promote clinical management strategies. Keywords: Renal cell carcinoma (RCC); renal tumor; immunohistochemistry; molecular genetics Submitted Apr 14, 2015. Accepted for publication Jan 15, 2016. doi: 10.3978/j.issn.1000-9604.2016.01.03 View this article at: http://dx.doi.org/10.3978/j.issn.1000-9604.2016.01.03 Introduction neoplasms and emerging/provisional new entities. -
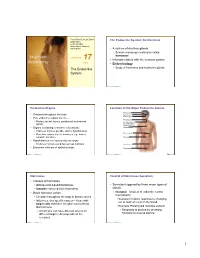
The Endocrine System
PowerPoint® Lecture Slides The Endocrine System: An Overview prepared by Leslie Hendon University of Alabama, Birmingham • A system of ductless glands • Secrete messenger molecules called hormones C H A P T E R 17 • Interacts closely with the nervous system Part 1 • Endocrinology The Endocrine • Study of hormones and endocrine glands System Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Endocrine Organs Location of the Major Endocrine Glands Pineal gland • Scattered throughout the body Hypothalamus Pituitary gland • Pure endocrine organs are the … Thyroid gland • Pituitary, pineal, thyroid, parathyroid, and adrenal Parathyroid glands glands (on dorsal aspect of thyroid gland) • Organs containing endocrine cells include: Thymus • Pancreas, thymus, gonads, and the hypothalamus Adrenal glands • Plus other organs secrete hormones (eg., kidney, stomach, intestine) Pancreas • Hypothalamus is a neuroendocrine organ • Produces hormones and has nervous functions Ovary (female) Endocrine cells are of epithelial origin • Testis (male) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Figure 17.1 Hormones Control of Hormones Secretion • Classes of hormones • Amino acid–based hormones • Secretion triggered by three major types of • Steroids—derived from cholesterol stimuli: • Basic hormone action • Humoral—simplest of endocrine control mechanisms • Circulate throughout the body in blood vessels • Secretion in direct response to changing • Influences only specific tissues— those with ion or nutrient levels in the blood target cells that have receptor molecules for that hormone • Example: Parathyroid monitors calcium • A hormone can have different effects on • Responds to decline by secreting different target cells (depends on the hormone to reverse decline receptor) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. -

Essentials of Abdominal Fine Needle Aspiration Cytology
1 ESSENTIALS OF ABDOMINAL FINE NEEDLE ASPIRATION CYTOLOGY Gia-Khanh Nguyen 2008 2 ESSENTIALS OF ABDOMINAL FINE NEEDLE ASPIRATION CYTOLOGY Gia-Khanh Nguyen, M.D. Professor Emeritus Laboratory Medicine and Pathology University Of Alberta Edmonton, Alberta, Canada Copyright by Gia-Khanh Nguyen Revised first edition, 2008 First edition, 2007. All rights reserved. This book was legally deposited at the Library and Archives Canada. ISNB: 0-9780929-2-9 3 TABLE OF CONTENTS Table of contents 3 Preface 4 Dedication 5 Related material 6 Key to abbreviations 7 Chapter 1. Pancreas and ampullary region 8 Chapter 2. Liver and biliary tree 39 Chapter 3. Kidney and renal pelvis 70 Chapter 4. Adrenal gland 87 Chapter 5. Other mass lesions 102 4 PREFACE The monograph “Essentials of Abdominal Fine Needle Aspiration Cytology” is written for practicing pathologists in community hospitals, residents in pathology and cytotechnologists who are interested in acquiring a basic knowledge on fine needle aspiration cytology of abdominal tumors/lesions. Commonly encountered tumors and uncommon lesions with characteristic cytologic manifestations are presented. Diagnostic criteria are presented and value and limitations of immunocytochemistry in tumor typing and differential diagnosis are stressed. For almost all lesions histopathologic images are included for cytohistologic correlation. Important references are listed in alphabetic order at the end of each chapter for further consultation. This monograph was prepared by myself. Therefore, a few typographical errors -

Corticotroph Hyperplasia and Cushing Disease: Diagnostic Features and Surgical Management
» This article has been updated from its originally published version to correct an error in the Discussion. See the corresponding erratum notice, DOI: 10.3171/2020.9.JNS201514a. « CLINICAL ARTICLE Corticotroph hyperplasia and Cushing disease: diagnostic features and surgical management Michael P. Catalino, MD, MSc,1,2 David M. Meredith, MD, PhD,3,4 Umberto De Girolami, MD,3,4 Sherwin Tavakol, MPH,1,5 Le Min, MD, PhD,6 and Edward R. Laws Jr., MD1,4 1Department of Neurosurgery, Brigham and Women’s Hospital/Harvard Medical School, Boston, Massachusetts; 2Department of Neurosurgery, University of North Carolina Hospitals, Chapel Hill, North Carolina; 3Department of Pathology, Brigham and Women’s Hospital/Harvard Medical School, Boston; 4Dana Farber Cancer Institute, Boston; 5Harvard TH Chan School of Public Health, Boston; and 6Division of Endocrinology, Brigham and Women’s Hospital/Harvard Medical School, Boston, Massachusetts OBJECTIVE This study was done to compare corticotroph hyperplasia and histopathologically proven adenomas in patients with Cushing disease by analyzing diagnostic features, surgical management, and clinical outcomes. METHODS Patients with suspected pituitary Cushing disease were included in a retrospective cohort study and were excluded if results of pathological analysis of the surgical specimen were nondiagnostic or normal. Cases were reviewed by two experienced neuropathologists. Total lesion removal was used as a dichotomized surgical variable; it was defined as an extracapsular resection (including a rim of normal gland) in patients with an adenoma, and for hyperplasia patients it was defined as removal of the presumed lesion plus a rim of surrounding normal gland. Bivariate and multivariate analyses were performed. Recurrence-free survival was compared between the two groups. -

The P300 and CBP Transcriptional Coactivators Are Required for Beta Cell and Alpha Cell
Page 1 of 46 Diabetes The p300 and CBP transcriptional coactivators are required for beta cell and alpha cell proliferation Chi Kin Wong1,2, Adam K Wade-Vallance2, Dan S Luciani2,3, Paul K Brindle4, Francis C Lynn2,3,5, William T Gibson1,2 1. Department of Medical Genetics, University of British Columbia, Vancouver, BC, Canada 2. BC Children’s Hospital Research Institute, Vancouver, BC, Canada 3. Department of Surgery, University of British Columbia, Vancouver, BC, Canada 4. St. Jude Children’s Research Hospital, Memphis, TN, USA 5. Departments of Cellular & Physiological Sciences, University of British Columbia, Vancouver, BC, Canada *Corresponding author: Chi Kin Wong, [email protected], (604)-875-2000 ext 6783 Running title: roles of p300/CBP in pancreatic islets Word counts: 4000 Number of table: 0 Number of figure: 6 Diabetes Publish Ahead of Print, published online December 7, 2017 Diabetes Page 2 of 46 Abstract p300 (EP300) and CBP (CREBBP) are transcriptional coactivators with histone acetyltransferase activity. Various beta cell transcription factors can recruit p300/CBP, and thus the coactivators could be important for beta cell function and health in vivo. We hypothesized that p300/CBP contribute to the development and proper function of pancreatic islets. To test this, we bred and studied mice lacking p300/CBP in their islets. Mice lacking either p300 or CBP in islets developed glucose intolerance attributable to impaired insulin secretion, together with reduced alpha and beta cell area and islet insulin content. These phenotypes were exacerbated in mice with only a single copy of p300 or CBP expressed in islets.