The Effect of Storage Temperature on the Composition of Fatty Acids in Crimson Sweet (Citrullus Lanatus Var
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Watermelon Seed Oil: Its Extraction, Analytical Studies, Modification and Utilization in Cosmetic Industries
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056 Volume: 07 Issue: 02 | Feb 2020 www.irjet.net p-ISSN: 2395-0072 Watermelon Seed Oil: Its Extraction, Analytical studies, Modification and Utilization in Cosmetic Industries Sarfaraz Athar1, Abullais Ghazi2, Osh Chourasiya3, Dr. Vijay Y. Karadbhajne4 1,2,3Department of Oil Technology, Laxminarayan Institute of Technology, Nagpur 4Head, Dept. of Oil Technology, Professor, Laxminarayan Institute of Technology, Nagpur, Maharashtra, India ---------------------------------------------------------------------***--------------------------------------------------------------------- Abstract - Watermelon seed is one of the unexplored seed in acid or omega 6 fatty acid (about 45-73%). Oleic, palmitic the world which is often discarded after eating the fruit. and stearic acid are also present in small quantities [3]. Researches show that these seeds contain nutrients like protein, essential fatty acids, vitamins and minerals. Oil Various researches report the positive effect of watermelon content in the seeds is between 35-40% and the unsaturated seed oil over skin. The oil is light, consists of humectants and fatty acid content in oil is 78-86% predominantly linoleic acid moisturising properties. It is easily absorbed by skin and (45-73%). This oil is effective for skin care as it is light, easily helps in restoring the elasticity of skin. Due to these absorbable and has humectants properties. Our study is about attributes this oil can be used in cosmetic industry for extraction of watermelon seed oil by solvent extraction process production of skin care products. The watermelon seed oil with the use of different solvents, its analysis and application can also be used as an anti inflammatory agent [4]. -

Natural, Vegan & Gluten-Free
Natural, Vegan & Gluten-Free All FarmHouse Fresh® products are Paraben and Sulfate free, made with up to 99.6% natural ingredients and made in Texas. Many of our products are also Vegan and Gluten-Free! Ingredient Decks Honey Heel Glaze: Water/Eau, Glycerine, Polysorbate 20, Polyquaternium-37, Parfum, Honey, Natural Rice Bran Oil (Orza Sativa), Tocopheryl Acetate (Vitamin E),Tetrahexyldecyl Ascorbate, Panthenol, Carica Papaya (Papaya) Fruit Extract, Ananas Sativus (Pineapple) Fruit Extract, Aloe Barbadensis Leaf Juice, Lactic Acid, PEG-40 Hydrogenated Castor Oil, Potassium Sorbate, Diazolidinyl Urea, DMDM Hydantoin, Caramel, Annatto. Strawberry Smash: Aloe Barbadensis Leaf Juice, Water/Eau, Glycerin, Glycine Soja (Soybean) Oil, Polysorbate 80, Butyrospermum Parkii (Shea Butter), Cetearyl Alcohol, Ceteareth-20, Cetyl Alcohol, Stearyl Alcohol, Parfum, Fragaria Vesca (Strawberry) Fruit Extract, Oryza Sativa (Rice) Barn Oil, Ethylhexyl Palmitate, Dimethicone, Cyanocobalmin (Vitamin B12), Tocopheryl Acetate, Menthone Glycerin Acetal, Carbomer, Disodium EDTA, Tetrasodium EDTA, Sodium Hydroxymethylglycinate, Sodium Hydroxide, Phenoxyethanol, Caprylyl Glycol, Potassium Sorbate Sweet Cream Body Milk: Water/Eau, Glycine Soja (Soybean) Oil, Sorbitol, Glycerin, Glyceryl Stearate, PEG-100 Stearate, Cetyl Alcohol, Oryza Sativa (Rice) Bran Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Persea Gratissima (Avocado) Oil, Sesamum Indicum (Sesame) Seed Oil, Stearyl Alcohol, Dimethicone, Polysorbate 20, Carbomer, -

KALAHARI MELON SEED Cold Pressed
Tel: +27(0)83 303 8253 ADDRESS: Vlakbult Tel: +27(0)827893035 PO Box 35 Clocolan [email protected] 9735 South Africa KALAHARI MELON SEED Cold pressed oil LATIN NAME: Citrullus lanatus INCI NAME: Citrullus lanatus (watermelon) seed oil OTHER NAMES: Karkoer, Mankataan, Tshamma, Ootanga White Watermelon, Wild Melon, Wild Watermelon CAS Nr: 90063-94-8 SOURCE: Cold pressed from the seed. COLOUR Colourless to very light yellow-green colour AROMA Very subtle neutral odour CULTIVATION: Commercially grown ORIGEN: South Africa It is the biological ancestor of the watermelon, which is now found all over the world, but which originated in the Kalahari region of Southern Africa. Unlike the common watermelon, whose flesh is sweet and red, the Kalahari melon’s flesh is pale yellow or green, and tastes OVERVIEW bitter. It is a creeping annual herb. The Kalahari melon has hairy stems, forked tendrils and three-lobed hairy leaves. Its flowers are bright yellow. The fruits vary significantly, from small and round in the wild, to larger and more oblong- shaped under cultivation. The surface is smooth, pale green with irregular bands of mottled darker green radiating from the stalk. The flesh is a pale green or yellow, and contains numerous brown seeds. In its wild form, the fruit is bitter to bland in taste, and largely inedible when fresh. The Kalahari melon or edible tsamma is ‘sweet' and highly adapted to surviving drought and the harsh light of the desert environment. Although found all over Southern Africa, it is most closely associated with the Kalahari sands of Namibia, Botswana, south-western Zambia and Bushmans eating Kalahari western Zimbabwe. -

Effect of Process Parameters on the Physical Properties of Watermelon Seed Oil Under Uniaxial Compression
Nutrition & Food Science International Journal ISSN 2474-767X Research Article Nutri Food Sci Int J - Volume 4 Issue 1 November 2017 Copyright © All rights are reserved by Nwosu Caesar DOI: 10.19080/NFSIJ.2017.04.555626 Effect of Process Parameters on the Physical Properties of Watermelon Seed Oil under Uniaxial Compression Nwosu Caesar1*, Ozumba Isaac C1 and Kabir Abdullahi O2 1Agro-Industrial Development and Extension Department, National Centre for Agricultural Mechanization, Nigeria 2Agricultural and Bio systems Engineering Department, University of Ilorin, Nigeria Submission: May 01, 2017; Published: November 17, 2017 *Corresponding author: Nwosu Caesar, Agro-Industrial Development and Extension Department, National Centre for Agricultural Mechanization, Nigeria, Email: Abstract The effect of process parameters such as temperature and pressure on the physical properties of watermelon seed oil under uniaxial compression was studied. A laboratory mechanical oil expression piston-cylinder rig was used to express oil from watermelon seeds at different temperatures (100 °C, 110 °C, 120 °C and 130 °C) and different pressures (5000Kg, 5500Kg, 6000Kg and 6500Kg). The expressed oil was analyzed analysis using Analysis of variance (ANOVA). for Viscosity, Specific gravity, Refractive Index and Boiling Point using standard methods. The results obtained were subjected to statistical The results revealed that viscosity of the expressed oil consistently decreased with increase in heating temperature for all the pressure regimes under study and increased with increasing applied pressure for all temperature regimes under study. The results also showed that wasprocess also conditions revealed in did this not study affect that the while specific increasing gravity temperatureof the expressed reduces oil in Boiling a particular point, increasingpattern. -

Date Seeds: a Promising Source of Oil with Functional Properties
foods Review Date Seeds: A Promising Source of Oil with Functional Properties Abdessalem Mrabet 1,2, Ana Jiménez-Araujo 1,* , Rafael Guillén-Bejarano 1 , Rocío Rodríguez-Arcos 1 and Marianne Sindic 2 1 Instituto de la Grasa, Consejo Superior de Investigaciones Científicas (CSIC), Campus Universitario Pablo de Olavide, Edificio 46, Ctra. de Utrera, 41013 Seville, Spain; [email protected] (A.M.); [email protected] (R.G.-B.); [email protected] (R.R.-A.) 2 Department Agro-Bio-Chem, University of Liege—Gembloux Agro-Bio Tech, Passage des Déportés 2, 5030 Gembloux, Belgium; [email protected] * Correspondence: [email protected] Received: 20 May 2020; Accepted: 11 June 2020; Published: 16 June 2020 Abstract: The cultivation of the date palm (Phoenix dactylifera L.) is the main activity and source of livelihood for people from arid and semiarid regions of the world. Date production is increasing every year. In addition, pitted date exportation is rising and great amounts of date seeds are produced. This biomass represents a problem for manufacturing companies. At the moment, date seeds are normally discarded or used as animal feed ingredients. However, this co-product can be used for many other applications due to its valuable chemical composition. Oil is one of the most interesting components of the date seed. In fact, date seeds contain 5–13% oil. Date seed oil contains saturated and unsaturated fatty acids with lauric and oleic as the main ones, respectively. Tocopherols, tocotrienols, phytosterols, and phenolic compounds are also present in significant amounts. These phytochemicals confer added value to date seed oil, which could be used for many applications, such as food product formulations, cosmetics, and pharmaceuticals. -

Menu Ingredient Statement
A&W Menu Ingredient Statement Vegan/ Menu Item Ingredients Allergen Sensitivities Vegetarian Burgers and Chicken Bun: Wheat Flour, Water, Corn Syrup, Soybean Oil, Yeast, Contains 2% or less of the following: Wheat Gluten, Salt, Malt, Dough Conditioners (Monoglycerides, Sodium Stearoyl Lactylate, Azodicarbonamide), Artificial Flavor, Yeast Nutrients (Calcium Sulfate, Ammonium Chloride), Calcium Propionate (Preservative). Beef Patty: Hamburger (ground beef). Seasoning: Salt, sugar, spices, paprika, dextrose, onion powder, corn starch, garlic powder, hydrolyzed cornprotein, extractive of paprika, disodium inosinate, disodium guanylate, silicon dioxide (anti-caking agent). Iceberg Lettuce Tomato Papa Burger/ Papa Egg, Milk, Yellow Onion Slices Gluten Burger Single Papa Sauce: Soybean oil, water, sugar, pickle relish (pickles, corn sweeteners, distilled vinegar, salt, xanthan gum, alum 0.10% potassium sorbate as a preservative, Wheat, Soy turmeric, natural spice flavors), tomato paste, distilled vinegar, egg yolk, salt, xanthan gum, artificial color including FD&C Red #40, spices, oleoresin paprika, Beta Apo-8-Carotenal, natural flavorings. Sharp American Cheese: Cultured milk and skim milk, water, cream, sodium citrate, salt, sorbic acid (preservative), sodium phosphate, artificial color, acetic acid, lecithin, enzymes. Pickle Slices: Pickles, water, distilled vinegar, salt, calcium chloride, potassium sorbate (as a preservative), polysorbate 80, natural flavors, turmeric oleoresin, garlic powder. Bun: Wheat Flour, Water, Corn Syrup, Soybean Oil, Yeast, Contains 2% or less of the following: Wheat Gluten, Salt, Malt, Dough Conditioners (Monoglycerides, Sodium Stearoyl Lactylate, Azodicarbonamide), Artificial Flavor, Yeast Nutrients (Calcium Sulfate, Ammonium Chloride), Calcium Propionate (Preservative). Beef Patty: Hamburger (ground beef). Seasoning: Salt, sugar, spices, paprika, dextrose, onion powder, corn starch, garlic powder, hydrolyzed cornprotein, extractive of paprika, disodium inosinate, disodium guanylate, silicon dioxide (anti-caking agent). -

The Soap Making Companion a Guide for Beginners 2Nd Edition
The Soap Making Companion A Guide for Beginners 2nd Edition The companion guidebook to the Thermal Mermaid Artisan Soap Makers Course By Thermal Mermaid & Jennifer Tynan Copyright 2020 by SpeckledEggPublishing All rights reserved. This book, or parts within may not be reproduced without permission from the publisher. Published by SpeckledEggPublishing Waterbury, CT 06706 https://speckledeggpublishing.com Contains material edited and modified from The Soap Making Companion: A Guide for Beginners, 1st Edition, by Jennifer Tynan, copyright 2018, 2015. ISBN: 9798643284789 Printed in the United States of America This publication is designed to provide accurate information in respect to the material presented. It is sold with the understanding that the recipes and suggestions are personal thoughts and understanding on the artisan craft of soap making and is not engaged in professional or medical advice. No claims are made about any of the recipes regarding cures, therapies, or treatments for personal care. Soap is made for cleaning, and the recipes in this book are geared toward the artistic techniques in creating only soap. Photography by Jennifer Tynan 10 9 8 7 6 5 4 3 2 2 Table of Contents Introduction 1. 1: Soaping, Setup, & Safety Welcome to the Beginning Gather the Supplies You Need Lye Safety & First Aid How to Mix & Use Lye Properly Saponification & Lye Calculations by Hand First Simple Soap Recipe 2. Cold Process Method, Techniques & Designs White Soap Trace Lavender Flowers Gel Insulating Glycerin Rivers Soda Ash Neapolitan Clay Bar Salt Bar & Brine Drop Swirl Soap 3 Hangar Swirl In the Bowl Swirl Column Pour Peacock Swirl Lava Bubbles 3. -
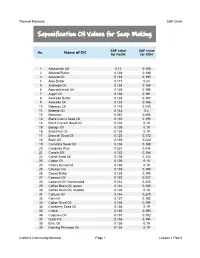
Saponification Oil Values for Soap Making
Thermal Mermaid SAP Chart Saponification Oil Values for Soap Making SAP value SAP value No. Name of Oil for NaOH for KOH 1 Abyssinian Oil 0.12 0.168 2 Almond Butter 0.134 0.188 3 Almond Oil 0.139 0.195 4 Aloe Butter 0.171 0.24 5 Andiroba Oil 0.134 0.188 6 Apricot Kernal Oil 0.139 0.195 7 Argan Oil 0.136 0.191 8 Avocado Butter 0.133 0.187 9 Avocado Oil 0.133 0.186 10 Babassu Oil 0.175 0.245 11 Baobab Oil 0.143 0.2 12 Beeswax 0.067 0.094 13 Black Cumin Seed Oil 0.139 0.195 14 Black Currant Seed Oil 0.135 0.19 15 Borage Oil 0.135 0.19 16 Brazil Nut Oil 0.135 0.19 17 Broccoli Seed Oil 0.123 0.172 18 Buriti Oil 0.159 0.223 19 Camelina Seed Oil 0.134 0.188 20 Candelila Wax 0.031 0.044 21 Canola Oil 0.133 0.186 22 Carrot Seed Oil 0.103 0.144 23 Castor Oil 0.128 0.18 24 Cherry Kernal Oil 0.135 0.19 25 Chicken Fat 0.139 0.195 26 Cocoa Butter 0.138 0.194 27 Coconut Oil 0.183 0.257 28 Coconut Oil, fractionated 0.232 0.325 29 Coffee Been Oil, green 0.132 0.185 30 Coffee Bean Oil, roasted 0.128 0.18 31 Cohune Oil 0.146 0.205 32 Corn Oil 0.137 0.192 33 Cotton Seed Oil 0.138 0.194 34 Cranberry Seed Oil 0.135 0.19 35 Crisco 0.138 0.193 36 Cupuacu Oil 0.137 0.192 37 Duck Fat 0.138 0.194 38 Emu Oil 0.135 0.19 39 Evening Primrose Oil 0.135 0.19 Crafter's Community Member Page 1 Lesson 1 Part 5 Thermal Mermaid SAP Chart SAP value SAP value No. -

Improvement in the Extraction of Hass Avocado Virgin Oil by Ultrasound Application
Journal of Food Research; Vol. 7, No. 2; 2018 ISSN 1927-0887 E-ISSN 1927-0895 Published by Canadian Center of Science and Education Improvement in the Extraction of Hass Avocado Virgin Oil by Ultrasound Application Nadia Segura1, Miguel Alejandro Amarillo1, Natalia Ines Martinez1 & María Antonia Grompone1 1Área Grasas y Aceites, Depto CYTAL, Facultad de Química, Univerisdad de la República, Montevideo, Uruguay Correspondence: Miguel Alejandro Amarillo. Área Grasas y Aceites, Depto CYTAL, Facultad de Química, Univerisdad de la República, Montevideo, Uruguay. E-mail: [email protected] Received: December 11, 2017 Accepted: December 29, 2017 Online Published: February 23, 2018 doi:10.5539/jfr.v7n2p106 URL: https://doi.org/10.5539/jfr.v7n2p106 Abstract The virgin oil extraction from avocado Hass was carried using an Abencor pilot scale plant. High-power ultrasound (1.73 MHz) was applied after mixing. High-frequency ultrasound device consists of two transductors which each deliver 30 W/L. Different proportions of water were added before ultrasound treatment (no water added and paste:water relation (1:3, 1:2, 1:1, 2:1, 3:1) and also different ultrasound application times (0, 1, 2, 3, 4, 5, 10, 15, 20 and 25 minutes). The study was carried out by setting one of the two variables considered and changing the values of the other. In the water addition study, ultrasound time was set at 15 min. It was found that oil recovery increased with the percentage of water added. It was decided to employ a ratio of 1:1 to study the influence of ultrasound application time. -
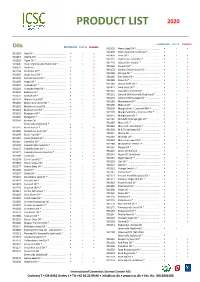
Product List 2020
PRODUCT LIST 2020 CONVENTIONAL ORGANIC STABILIZED Oils CONVENTIONAL ORGANIC STABILIZED 901199 Hemp Seed Oil * .................................. • • • 901499 Hemp Seed Oil Unrefined * ................. • • 901193 Acai Oil * ............................................. • • • 901450 Inchi Oil *............................................. • • • 901367 Alfalfa Oil* ........................................... • • • 901112 Jojoba Oil – Colorless * ........................ • • • 901228 Algae Oil * ........................................... • • • 901110 Jojoba Oil – Golden * ........................... • • • 907440 Aloe Oil (Internally Stabilized)* .......... • • 901162 Kakadu Oil * ........................................ • • • 906221 Amla Oil .............................................. • • 901152 Kalahari Melon Seed Oil * ................... • • • 901148 Andiroba Oil * ..................................... • • • 901168 Karanja Oil * ........................................ • • • 901387 Apple Seed Oil * .................................. • • • 901165 Kiwi Seed Oil * ..................................... • • • 901176 Apricot Kernel Oil * ............................. • • • 901185 Kukui Oil * ........................................... • • • 901195 Argan Oil * ........................................... • • • 901180 Lemon Seed Oil * ................................ • • • 901118 Avocado Oil * ...................................... • • • 901421 Lime Seed Oil * .................................... • • • 901218 Avocado Seed Oil * ............................. -

Quantification of Rice Bran Oil in Oil Blends
GRASAS Y ACEITES, 63 (1), ENERO-MARZO, 53-60, 2012, ISSN: 0017-3495 DOI: 10.3989/gya.033311 Quantification of rice bran oil in oil blends By R. Mishra*, H.K. Sharma and G. Sengar Food Engineering and Technology Department. Sant Longowal Institute of Engineering & Technology (Deemed to be University) Longowal – 148 106. Sangrur (PUNJAB). INDIA *Corresponding author: [email protected] RESUMEN ultrasonic velocity and methods based on physico-chemical parameters. The physicochemical parameters such as Cuantificación de aceite de salvado de arroz en mez- ultrasonic velocity, relative association and acoustic clas de aceites. impedance at 2 MHz, iodine value, palmitic acid content and oryzanol content reflected significant changes with Se analizaron diversos parámetros físico-químicos pa- increased proportions of PRBO in the blended oils. These ra la evaluación de mezclas de aceites en diferentes pro- parameters were selected as dependent parameters and % porciones que incluyen: aceite de salvado de arroz físíca- PRBO proportion was selected as independent parameters. mente refinado (PRBO): aceite de girasol (SNF) y las The study revealed that regression equations based on mezclas PRBO: aceite de cártamo (SAF) en diferentes pro- the oryzanol content, palmitic acid composition, ultrasonic porciones. La cuantificación de la presencia del aceite de velocity, relative association, acoustic impedance, and salvado de arroz en las mezclas se llevó a cabo por dife- iodine value can be used for the quantification of rice bran rentes métodos, como cromatografía de gases (GC), cro- oil in blended oils. The rice bran oil can easily be quantified matografía líquida (HPLC), ultrasonidos y métodos basa- in the blended oils based on the oryzanol content by HPLC dos en otros parámetros físico-químicos. -

Characterization of Seed Oil from Citrullus Lanatus (Watermelon)
Vol.3 (2), pp 34-40, May 2018 ISSN 4372-2603 DOI: https://doi.org/10.26765/DRJPHET.2018.7842 Article Number: DRJA6513097842 Copyright © 2018 Author(s) retain the copyright of this article Direct Research Journal of Public Health and Environmental Technology http://directresearchpublisher.org/journal/drjphet/ Research Paper Characterization of Seed Oil from Citrullus lanatus (Watermelon) Gabriel A. F., Igwemmar N. C, *Sadam A. A., and Babalola S. A. Department of Chemistry, Faculty of Science, University of Abuja, Abuja, Federal Capital Territory, Nigeria. *Corresponding author E-mail: [email protected]. Received 20 April 2018; Accepted 17 May, 2018 Large varieties of the pumpkin family such as watermelon seed oil detected in the sample using n-hexane and ethanol solvent. There have been previously explored for their medicinal conventions are major elements such as Zn (4.8 mg/100g), Ca (27.0 mg/100g) due to the presence of bioactive metabolites such as cucurbitacin, and Mg (29.8 mg/100g). Furthermore, the proximate analysis triterpenes, sterols, alkaloids, vitamins and minerals, but none of shows that there are presence of crude protein (19.43±0.38%), these products have gained utilization on industrial scale. This ash (5.03±0.05%) crude fibre (26.10±0.03%), crude lipid research is to verify the ongoing experimental and biological (21.54±0.2%), moisture (10.92±0.02%) and carbohydrate analysis to corroborate its ethno-medicinal utilization. It centers (16.98%) respectively. Although there have been significant on the investigation of the phytochemical composition and nutritional value in the seed which is the parts always subverted nutritional bioassay of the indigenous watermelon (Citrullus on the basis of acclaimed toxicity, these in turns can be lanatus ) seed oil sold in Gwagwalada market, Gwagwalada Abuja recommended on daily allowance, maintenance of good district Nigeria (Longitude 8°N and 7°E).