Factors Influencing Storage Quality of Hazelnut Varieties
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hazelnut: a Valuable Resource
International Journal of Food Engineering Vol. 6, No. 2, December 2020 Hazelnut: A Valuable Resource Raquel P. F. Guiné and Paula M. Reis Correia CI&DETS/ESAV, Polytechnic Institute of Viseu, Department of Food Industry, Viseu, Portugal Email: [email protected], [email protected] Abstract—Hazel (Corylus avellana L.) nutshell is one of the remaining 90% is used for industrial purposes as shelled most consumed and most appreciated nut fruit all over the nuts [7], [8]. world. It is believed to have constituted a basic food in early Similarly, to other nuts, hazelnuts are included in the prehistory, in temperate zones of the globe, such as for dietary guidelines of several countries, due to their high example Europe. Presently the hazelnut production is nutritional content and bioavailability of those nutrients. mainly concentrated on the Black Sea coast of Turkey, but other countries are also important producers, like for Furthermore, the bioactive molecules present in hazelnuts example Portugal, situated on the western Europe, in the have been reported as having several benefits for the Iberian Peninsula. The objective of this work is to make a human health [9]–[11]. review about the worldwide importance of hazelnut, their usages, including gastronomic and industrial applications, II. CONSUMPTION & MAIN USAGES as well as some ways that allow adding value to this fruit, making it an even more valuable resource. The advantages From the Neolithic era, in the regions of Europe and include higher income for produces, lower environmental the Caucasus, hazelnut has been used for human impacts and valorisation of residues improving consumption. -
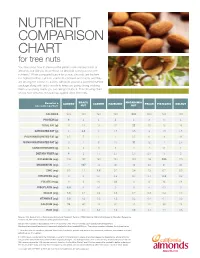
Nutrient Comparison Chart
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

List of Legumes
Healthy Oils & SmokePoints When it comes to the cooking oil in your cupboard, there is a huge difference in healthfulness depending on how the oil is stored and how it will be used. First let’s get some definitions for commonly used terms on labels and discussions about oils. Term Definition Cold Pressed Extracted without using heat. Extracted using a screw-type machine that presses the oil out. Can be done Expeller Pressed slowly, with very little heat, or can be done quickly with lots of friction and high temperatures. The first cold pressing which contains the best tasting and most healthful oils. Must contain less than 1% acids. By definition, this is cold-pressed and first Extra Virgin pressed, so don’t need to see these terms on the label. Must say 100% extra virgin, or may be a blend. The first cold pressing, but can contain a little more acids than the extra-virgin Virgin (1-3 percent). Seeds that have been genetically manipulated to decrease the amount of High Oleic essential fatty acids so that they have a longer shelf life. Are left in their state after pressing – no filtering. These oils tend to be more Unrefined Oils flavorful and richer in nutrients, however they have a very low smoke point. Oils have their impurities filtered out, to increase stability and allow for higher Refined temperature cooking. The processing can use toxic solvents, caustic soda, bleaches and phosphoric acid. Smoke Point Stage at which a heated oil begins to smoke, just before it bursts into flames. HEALTHY OILS & SMOKE POINTS PAGE | 1 © 2021 Health-Naturally.org Term Definition Oil should smell and taste like the food it came from. -

Pistachio Oil
Pistachio Oil Oleum Pistaciae synonyms: Pistazien(kern)öl (D); huile de pistache (F) 1 Source Plant Pistacia vera L. (Anacardiaceae), pistachio © Springer Nature Switzerland AG 2020 593 S. Krist, Vegetable Fats and Oils, https://doi.org/10.1007/978-3-030-30314-3_94 594 Pistachio Oil Habitat P Pistacia vera originates in Central Asia and the Mediterranean area. Alexander the Great brought it to Greece, and the Romans brought to to Sicily. Even today, pista- chios grow wild in several countries, for example in Afghanistan and India. They prefer dry, desert-like regions and are very frost-susceptible. Pistacia vera has a biennial crop sequence, which is why there are in turn small and large amounts of crop (Hager 1978, volume 6a, p. 730; Roth and Kormann 2000, p. 145). Description Pistacia vera is an evergreen, deciduous tree that can reach a height of 8–12 m. The crown is spreading and forms a dense canopy of leaves. The leaves are pinnate or bipinnate, greyish green, with stalkless, ovate leaflets. The panicle is short; the inconspicuous, axillary flowers are a reddish colour. They develop into elongated, oval stone fruit that are about 2–3 cm long. The fruit are brownish red and wrinkly, with a thin layer of fruit pulp that tastes of turpentine. They contain the seeds that are sold as pistachios or “green almonds”. The seed is usually triangular, a green, brownish or violet colour and 20 mm long. It is slightly compressed at the sides and protected by a whitish, hard shell (Hager 1978, volume 6a, p. -

From Olive to Olive Oil: a General Approach Da Oliveira Ao Azeite De Oliva: Uma Abordagem Geral De Oliveira Al Aceite De Oliva: Un Enfoque General
Research, Society and Development, v. 10, n. 3, e32210313408, 2021 (CC BY 4.0) | ISSN 2525-3409 | DOI: http://dx.doi.org/10.33448/rsd-v10i3.13408 From olive to olive oil: a general approach Da oliveira ao azeite de oliva: uma abordagem geral De oliveira al aceite de oliva: un enfoque general Received: 02/28/2021 | Reviewed: 03/08/2021 | Accept: 03/09/2021 | Published: 03/17/2021 Bruna Sanches Silva ORCID: https://orcid.org/0000-0003-0044-2282 Universidade Federal dos Vales do Jequitinhonha e Mucuri, Brazil E-mail: [email protected] Marcio Schmiele ORCID: https://orcid.org/0000-0001-8830-1710 Universidade Federal dos Vales do Jequitinhonha e Mucuri, Brazil E-mail: [email protected] Abstract This study aimed to carry out a systematic literature review about olives, extraction methods, physical and chemical characterization and identity and quality parameters of olive oils, as well as technological alternatives for using by- products. Olive oil is the oil extracted from the ripe fruits of the olive tree (Olea europaea L.). Trees have been cultivated in the Mediterranean Region for several centuries and thousands of cultivars differ by weight, size and chemical characteristics of the fruits. Currently, olive oil is produced worldwide and the olive plant was recently introduced in the city of Diamantina, Minas Gerais. The lipid content is mostly composed of oleic acid and smaller fractions of phenolic compounds, phytosterols and pigments, substances with antioxidant and bioactive activities that promote oxidative stability of the oil and beneficial effects on human health. The main extraction of olive oil consists of crushing, pressing and centrifuging, generating by-products that can be reused for recovery of compounds or generation of new products in the food industry. -

Watermelon Seed Oil: Its Extraction, Analytical Studies, Modification and Utilization in Cosmetic Industries
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056 Volume: 07 Issue: 02 | Feb 2020 www.irjet.net p-ISSN: 2395-0072 Watermelon Seed Oil: Its Extraction, Analytical studies, Modification and Utilization in Cosmetic Industries Sarfaraz Athar1, Abullais Ghazi2, Osh Chourasiya3, Dr. Vijay Y. Karadbhajne4 1,2,3Department of Oil Technology, Laxminarayan Institute of Technology, Nagpur 4Head, Dept. of Oil Technology, Professor, Laxminarayan Institute of Technology, Nagpur, Maharashtra, India ---------------------------------------------------------------------***--------------------------------------------------------------------- Abstract - Watermelon seed is one of the unexplored seed in acid or omega 6 fatty acid (about 45-73%). Oleic, palmitic the world which is often discarded after eating the fruit. and stearic acid are also present in small quantities [3]. Researches show that these seeds contain nutrients like protein, essential fatty acids, vitamins and minerals. Oil Various researches report the positive effect of watermelon content in the seeds is between 35-40% and the unsaturated seed oil over skin. The oil is light, consists of humectants and fatty acid content in oil is 78-86% predominantly linoleic acid moisturising properties. It is easily absorbed by skin and (45-73%). This oil is effective for skin care as it is light, easily helps in restoring the elasticity of skin. Due to these absorbable and has humectants properties. Our study is about attributes this oil can be used in cosmetic industry for extraction of watermelon seed oil by solvent extraction process production of skin care products. The watermelon seed oil with the use of different solvents, its analysis and application can also be used as an anti inflammatory agent [4]. -

Cod Liver Oil (Lemon Flavor)
Manufacturers.of.. Hypo-al.ler.gen.ic.. Nutritional.. Sup.ple.ments Cod Liver Oil (lemon flavor) INTRODUCED 1996 What Is It? from cod. It has been molecularly distilled and tested for heavy metals, pesticides, PCBs, dioxins and furans, Ultra-pure, molecularly distilled genuine Norwegian cod and other contaminants. Natural flavor is derived from liver oil provides essential omega-3 fatty acids along with lemon. natural vitamin A and D for immune, joint, cardiovascular and emotional health.* Recommendations Uses For Cod Liver Oil Pure Encapsulations recommends 1 or more teaspoons per day, with or between meals. Immune Health: Cod liver oil has a long history of traditional use for supporting immune health, primar- Are There Any Potential Side Effects Or ily attributed to vitamins A and D. Vitamin D receptors are found on most immune cells, including monocytes, Precautions? T cells and B cells and research suggests that it has an Cod liver oil may cause burping, gastrointestinal upset or immunomodulatory effect. Additionally, the essential fatty indigestion, nausea, diarrhea, or abdominal bloating. If acids in cod liver oil help promote a healthy inflammatory pregnant or lactating, consult your physician before taking response.* this product. Promotes Heart Health: EPA and DHA from cod liver oil encourage cardiovascular health by supporting Are There Any Potential Drug Interactions? triglyceride and lipid metabolism, maintaining healthy Cod liver oil may be contra-indicated with blood blood flow and promoting healthy platelet function. In thinning medications. Consult your physician for more addition, studies indicate these essential fatty acids help information. maintain blood vessel integrity. One study also suggests EPA may moderate protein kinase C, supporting arterial Cod Liver Oil (lemon flavor) smooth muscle cells.* each teaspoon (5 ml) provides Supports Joint Health: Cod liver oil is important for optimal joint function. -

Tocochromanols: Rancid Lipids, Seed Longevity, and Beyond
COMMENTARY Tocochromanols: Rancid lipids, seed longevity, and beyond Nicholas Smirnoff1 College of Life and Environmental Sciences, University of Exeter, Exeter EX4 4QD, United Kingdom nvestigation of tocochromanols, li- pophilic molecules that protect I against fatty acid peroxidation, is still providing surprises. They are uniquely synthesized by photosynthetic organisms, widely studied in relation to their photoprotective role in photosyn- thesis, and are of importance in human nutrition as vitamin E. An article in PNAS from the DellaPenna laboratory (1) makes a significant step forward by showing that tocochromanols are essential for main- taining the viability of Arabidopsis thaliana seeds, suggesting that tocochromanols are a key component in the evolution of des- iccation-resistant seeds. Tocochromanols Protect Against Lipid Peroxidation Lipid peroxidation is a chain reaction in which unsaturated fatty acids in lipids of Fig. 1. Peroxidation of an unsaturated fatty acyl chain is initiated by a hydroxyl radical (OH·)oranacyl various types are oxidized, producing peroxyl radical (R·). Tocochromanols scavenge acyl peroxyl radicals and therefore minimize their reaction highly reactive products that can cause with further acyl chains. Details of the tocochromanol oxidation products are not shown, but most of further cellular damage. Polyunsaturated these can be converted back to tocochromanol. fatty acids (PUFAs) are particularly sus- ceptible to peroxidation, a chain reaction active oxygen species formed during pho- PC-8. The double vte1 vte2 mutant lacks initiated by hydroxyl radicals (Fig. 1). tosynthesis (7). both tocopherols and PC-8 (1). Its seeds The first reasonably stable products, fatty rapidly lose viability after harvesting owing acyl hydroperoxides, are still sufficiently Rancid Lipids and Seed Longevity to catastrophic fatty acid peroxidation, reactive to produce aldehydes (reactive The article by Mène-Saffrané et al. -

Natural, Vegan & Gluten-Free
Natural, Vegan & Gluten-Free All FarmHouse Fresh® products are Paraben and Sulfate free, made with up to 99.6% natural ingredients and made in Texas. Many of our products are also Vegan and Gluten-Free! Ingredient Decks Honey Heel Glaze: Water/Eau, Glycerine, Polysorbate 20, Polyquaternium-37, Parfum, Honey, Natural Rice Bran Oil (Orza Sativa), Tocopheryl Acetate (Vitamin E),Tetrahexyldecyl Ascorbate, Panthenol, Carica Papaya (Papaya) Fruit Extract, Ananas Sativus (Pineapple) Fruit Extract, Aloe Barbadensis Leaf Juice, Lactic Acid, PEG-40 Hydrogenated Castor Oil, Potassium Sorbate, Diazolidinyl Urea, DMDM Hydantoin, Caramel, Annatto. Strawberry Smash: Aloe Barbadensis Leaf Juice, Water/Eau, Glycerin, Glycine Soja (Soybean) Oil, Polysorbate 80, Butyrospermum Parkii (Shea Butter), Cetearyl Alcohol, Ceteareth-20, Cetyl Alcohol, Stearyl Alcohol, Parfum, Fragaria Vesca (Strawberry) Fruit Extract, Oryza Sativa (Rice) Barn Oil, Ethylhexyl Palmitate, Dimethicone, Cyanocobalmin (Vitamin B12), Tocopheryl Acetate, Menthone Glycerin Acetal, Carbomer, Disodium EDTA, Tetrasodium EDTA, Sodium Hydroxymethylglycinate, Sodium Hydroxide, Phenoxyethanol, Caprylyl Glycol, Potassium Sorbate Sweet Cream Body Milk: Water/Eau, Glycine Soja (Soybean) Oil, Sorbitol, Glycerin, Glyceryl Stearate, PEG-100 Stearate, Cetyl Alcohol, Oryza Sativa (Rice) Bran Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Persea Gratissima (Avocado) Oil, Sesamum Indicum (Sesame) Seed Oil, Stearyl Alcohol, Dimethicone, Polysorbate 20, Carbomer, -

Rabbi Eliyahu Shuman Director of Supervision Effective Through 11
June 2, 2021 22 Sivan 5781 La Tourangelle, Inc. 2340 East Main Street Suite 400 Woodland, CA 95776 This is to certify that the 39 products specified in the listing below, manufactured by LA TOURANGELLE, INC. of the above address, are Kosher and under our supervision. PLEASE NOTE THE FOLLOWING CONDITIONS OF CERTIFICATION: All products listed below are Pareve. All products listed below are certified only when bearing the Star-K symbol. All products listed below are Kosher for year-round use, excluding Passover. This letter of certification is valid through November 30, 2021 and is subject to renewal at that time. BRAND: La Tourangelle UKD# PRODUCT LISTING SK0HZG4N550 All Purpose Baking Spray SKDZZE3SG6W Avocado Oil SKVLKXREYAU Avocado Oil Non-Stick Cooking Spray SKB0NQF2TZU AvoCoco Oil SK5KPHXJ7WR Everyday Avocado Oil SK27270H2EA Expeller Pressed Vegetable Oil SK3YJF27B06 Grape Seed Oil SKZR5EBNPJE Grapeseed Oil Non-Stick Cooking Spray SKTA059CQ2D Organic Canola Oil SKEVY33L343 Organic Canola Oil Non-Stick Cooking Spray SKK08UW6IKZ Organic Expeller Pressed & Refined Coconut Oil SKAC2BUNNSR Organic Extra Virgin Coconut Oil SKN6ZPNS4QU Organic Extra Virgin Olive Oil SKIVAMH6Y1J Organic Extra Virgin Olive Oil Non-Stick Cooking Spray SKYKMZA08PT Organic Flaxseed Oil SKSDDTU4VXP Organic Refined Coconut Oil SK8C58TF7N1 Organic Sun Coco Oil SKGNEZ4A2KZ Organic Sunflower Oil SKR3YHL7L04 Organic Toasted Sunflower Oil SKWVAF83EW0 Organic Virgin Unrefined Coconut Oil SKQ4DM4KBR7 Pan Asian Stir Fry Oil Rabbi Eliyahu Shuman Effective Through 11/30/2021 Director of Supervision Page 1 of 2 June 2, 2021 22 Sivan 5781 La Tourangelle, Inc. 2340 East Main Street Suite 400 Woodland, CA 95776 PLEASE NOTE THE FOLLOWING CONDITIONS OF CERTIFICATION: All products listed below are Pareve. -

Heart Health Through Whole Foods
Heart Health Through Whole Foods Certain whole foods in a diet can ultimately provide heart-healthy benefits. The right foods consumed in the right amounts can help lower cholesterol and/or triglycerides. They may also help to reduce risk for heart disease. Even though the benefits of whole foods may be known, too often individuals turn to over-the-counter supplements instead. It is important to discuss all supplements prior to ingestion with your physician. Individuals may not realize that taking some supplements with certain medications may be harmful or that taking too much of a good thing can be bad. The purpose of this session is to educate how to obtain certain nutrients through whole foods rather then through supplements. It must be noted that some individuals may still need supplements in addition to diet. Once again this should be guided by a physician. Supplement Health Benefits Caution Dietary Alternative Omega-3 Fatty Acids: Fish oil is used for There are some safety concerns Consuming fish oil from dietary Fish Oils reduction in cholesterol about using high doses of fish oil. sources such as fatty fish (e.g., and triglycerides. It is Doses greater than 3 grams per tuna, salmon), two servings Fish oils contain used for hyperlipidemia, day can inhibit blood coagulation per week, is associated with Eicosapentaenoic hypertriglyceridemia, and potentially increase the risk a reduced risk of developing Acid (EPA) and coronary heart disease of bleeding. Doses greater than 3 cardiovascular disease Docosahexaenoic and hypertension. grams per day might also suppress (primary prevention). Acid (DHA) immune response. -

KALAHARI MELON SEED Cold Pressed
Tel: +27(0)83 303 8253 ADDRESS: Vlakbult Tel: +27(0)827893035 PO Box 35 Clocolan [email protected] 9735 South Africa KALAHARI MELON SEED Cold pressed oil LATIN NAME: Citrullus lanatus INCI NAME: Citrullus lanatus (watermelon) seed oil OTHER NAMES: Karkoer, Mankataan, Tshamma, Ootanga White Watermelon, Wild Melon, Wild Watermelon CAS Nr: 90063-94-8 SOURCE: Cold pressed from the seed. COLOUR Colourless to very light yellow-green colour AROMA Very subtle neutral odour CULTIVATION: Commercially grown ORIGEN: South Africa It is the biological ancestor of the watermelon, which is now found all over the world, but which originated in the Kalahari region of Southern Africa. Unlike the common watermelon, whose flesh is sweet and red, the Kalahari melon’s flesh is pale yellow or green, and tastes OVERVIEW bitter. It is a creeping annual herb. The Kalahari melon has hairy stems, forked tendrils and three-lobed hairy leaves. Its flowers are bright yellow. The fruits vary significantly, from small and round in the wild, to larger and more oblong- shaped under cultivation. The surface is smooth, pale green with irregular bands of mottled darker green radiating from the stalk. The flesh is a pale green or yellow, and contains numerous brown seeds. In its wild form, the fruit is bitter to bland in taste, and largely inedible when fresh. The Kalahari melon or edible tsamma is ‘sweet' and highly adapted to surviving drought and the harsh light of the desert environment. Although found all over Southern Africa, it is most closely associated with the Kalahari sands of Namibia, Botswana, south-western Zambia and Bushmans eating Kalahari western Zimbabwe.