Paracoccus Chinensis Sp. Nov., Isolated from Sediment of a Reservoir
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

APP201895 APP201895__Appli
APPLICATION FORM DETERMINATION Determine if an organism is a new organism under the Hazardous Substances and New Organisms Act 1996 Send by post to: Environmental Protection Authority, Private Bag 63002, Wellington 6140 OR email to: [email protected] Application number APP201895 Applicant Neil Pritchard Key contact NPN Ltd www.epa.govt.nz 2 Application to determine if an organism is a new organism Important This application form is used to determine if an organism is a new organism. If you need help to complete this form, please look at our website (www.epa.govt.nz) or email us at [email protected]. This application form will be made publicly available so any confidential information must be collated in a separate labelled appendix. The fee for this application can be found on our website at www.epa.govt.nz. This form was approved on 1 May 2012. May 2012 EPA0159 3 Application to determine if an organism is a new organism 1. Information about the new organism What is the name of the new organism? Briefly describe the biology of the organism. Is it a genetically modified organism? Pseudomonas monteilii Kingdom: Bacteria Phylum: Proteobacteria Class: Gamma Proteobacteria Order: Pseudomonadales Family: Pseudomonadaceae Genus: Pseudomonas Species: Pseudomonas monteilii Elomari et al., 1997 Binomial name: Pseudomonas monteilii Elomari et al., 1997. Pseudomonas monteilii is a Gram-negative, rod- shaped, motile bacterium isolated from human bronchial aspirate (Elomari et al 1997). They are incapable of liquefing gelatin. They grow at 10°C but not at 41°C, produce fluorescent pigments, catalase, and cytochrome oxidase, and possesse the arginine dihydrolase system. -

A Salt Lake Extremophile, Paracoccus Bogoriensis Sp. Nov., Efficiently Produces Xanthophyll Carotenoids
African Journal of Microbiology Research Vol. 3(8) pp. 426-433 August, 2009 Available online http://www.academicjournals.org/ajmr ISSN 1996-0808 ©2009 Academic Journals Full Length Research Paper A salt lake extremophile, Paracoccus bogoriensis sp. nov., efficiently produces xanthophyll carotenoids George O. Osanjo1*, Elizabeth W. Muthike2, Leah Tsuma3, Michael W. Okoth2, Wallace D. Bulimo3, Heinrich Lünsdorf4, Wolf-Rainer Abraham4, Michel Dion5, Kenneth N. Timmis4 , Peter N. Golyshin4 and Francis J. Mulaa3 1School of Pharmacy, University of Nairobi, P. O. Box 30197-00100, Nairobi, Kenya. 2Department of Food Science, Technology and Nutrition, University of Nairobi, P.O. Box 30197-00100, Nairobi, Kenya. 3Department of Biochemistry, University of Nairobi, P. O. Box 30197-00100, Nairobi, Kenya. 4Division of Microbiology, Helmholtz Centre for Infection Research, Inhoffenstrasse 7, D-38124 Braunschweig, Germany. 5Université de Nantes, UMR CNRS 6204, Biotechnologie, Biocatalyse, Biorégulation, Faculté des Sciences et des Techniques, 2, rue de la Houssinière, BP 92208, Nantes, F- 44322, France. Accepted 27 July, 2009 A Gram-negative obligate alkaliphilic bacterium (BOG6T) that secretes carotenoids was isolated from the outflow of Lake Bogoria hot spring located in the Kenyan Rift Valley. The bacterium is motile by means of a polar flagellum, and forms red colonies due to the production of xanthophyll carotenoid pigments. 16S rRNA gene sequence analysis showed this strain to cluster phylogenetically within the genus Paracoccus. Strain BOG6T is aerobic, positive for both catalase and oxidase, and non- methylotrophic. The major fatty acid of the isolate is C18: 1ω7c. It accumulated polyhydroxybutyrate granules. Strain BOG6T gave astaxanthin yield of 0.4 mg/g of wet cells indicating a potential for application in commercial production of carotenoids. -

Characterization of Bacterial Communities Associated
www.nature.com/scientificreports OPEN Characterization of bacterial communities associated with blood‑fed and starved tropical bed bugs, Cimex hemipterus (F.) (Hemiptera): a high throughput metabarcoding analysis Li Lim & Abdul Hafz Ab Majid* With the development of new metagenomic techniques, the microbial community structure of common bed bugs, Cimex lectularius, is well‑studied, while information regarding the constituents of the bacterial communities associated with tropical bed bugs, Cimex hemipterus, is lacking. In this study, the bacteria communities in the blood‑fed and starved tropical bed bugs were analysed and characterized by amplifying the v3‑v4 hypervariable region of the 16S rRNA gene region, followed by MiSeq Illumina sequencing. Across all samples, Proteobacteria made up more than 99% of the microbial community. An alpha‑proteobacterium Wolbachia and gamma‑proteobacterium, including Dickeya chrysanthemi and Pseudomonas, were the dominant OTUs at the genus level. Although the dominant OTUs of bacterial communities of blood‑fed and starved bed bugs were the same, bacterial genera present in lower numbers were varied. The bacteria load in starved bed bugs was also higher than blood‑fed bed bugs. Cimex hemipterus Fabricus (Hemiptera), also known as tropical bed bugs, is an obligate blood-feeding insect throughout their entire developmental cycle, has made a recent resurgence probably due to increased worldwide travel, climate change, and resistance to insecticides1–3. Distribution of tropical bed bugs is inclined to tropical regions, and infestation usually occurs in human dwellings such as dormitories and hotels 1,2. Bed bugs are a nuisance pest to humans as people that are bitten by this insect may experience allergic reactions, iron defciency, and secondary bacterial infection from bite sores4,5. -

Sulphur Oxidising Bacteria in Mangrove Ecosystem: a Review
Vol. 13(29), pp. 2897-2907, 16 July, 2014 DOI: 10.5897/AJB2013.13327 Article Number: D2A2A3546087 ISSN 1684-5315 African Journal of Biotechnology Copyright © 2014 Author(s) retain the copyright of this article http://www.academicjournals.org/AJB Review Sulphur oxidising bacteria in mangrove ecosystem: A review B. C. Behera1, R. R. Mishra2, S. K. Dutta3 and H. N. Thatoi4* 1Department of Biotechnology, North Odisha University, Baripada -757003, Odisha, India. 2Department of Biotechnology, MITS School of Biotechnology, Bhubaneswar-751024, Odisha, India. 3Centre for Ecological Sciences, Indian Institute of Science, Bangalore - 560012, India. 4Department of Biotechnology, College of Engineering and Technology, Biju Pattnaik University of Technology, Bhubaneswar -751003, Odisha, India. Received 29 September, 2013; Accepted 16 June, 2014 Mangrove soils are anoxic, sulphidic and variable since their chemistry is regulated by a variety of factors such as texture, tidal range and elevation, redox state, bioturbation intensity, forest type, temperature and rainfall. Sulphur-oxidizing bacteria such as photoautotrophs, chemolithotrophs and heterotrophs play an important role in the mangrove environment for the oxidation of the toxic sulphide produced by sulphur reducing bacteria and act as a key driving force behind all sulphur transformations in the mangrove ecosystem which is most essential to maintain the sulphur cycle as well as eco health. These overviews summarizes the current state of knowledge of diversity and important biotechnological contributions of these microorganisms in agriculture, bio fertility, reduction of environmental pollution, maintenance of the productivity of ecosystems and also highlight areas in which further research is needed to increase our basic understanding of physiology, genomics and proteomics of these microorganisms which is most essential. -
![A Soil Actinobacterium Scavenges Atmospheric H2 Using Two Membrane-Associated, Oxygen-Dependent [Nife] Hydrogenases](https://docslib.b-cdn.net/cover/8907/a-soil-actinobacterium-scavenges-atmospheric-h2-using-two-membrane-associated-oxygen-dependent-nife-hydrogenases-1448907.webp)
A Soil Actinobacterium Scavenges Atmospheric H2 Using Two Membrane-Associated, Oxygen-Dependent [Nife] Hydrogenases
A soil actinobacterium scavenges atmospheric H2 using two membrane-associated, oxygen-dependent [NiFe] hydrogenases Chris Greeninga,b, Michael Berneya,c, Kiel Hardsa, Gregory M. Cooka,1, and Ralf Conradb,1 aDepartment of Microbiology and Immunology, University of Otago, Dunedin 9016, New Zealand; bMax-Planck Institute for Terrestrial Microbiology, D-35043 Marburg, Germany; and cDepartment of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, NY 10461 Edited by Edward F. DeLong, Massachusetts Institute of Technology, Cambridge, MA, and approved January 23, 2014 (received for review November 1, 2013) In the Earth’s lower atmosphere, H2 is maintained at trace concen- The majority of characterized hydrogen-oxidizing soil bacteria trations (0.53 ppmv/0.40 nM) and rapidly turned over (lifetime ≤ (e.g., Paracoccus denitrificans, Ralstonia eutropha) have a low −1 2.1 y ). It is thought that soil microbes, likely actinomycetes, serve affinity for H2 (Km ≥ 1 μM, threshold ≥ 1 nM) and hence are as the main global sink for tropospheric H2. However, no study has probably responsible for the former process; their uptake ever unambiguously proven that a hydrogenase can oxidize this hydrogenases (principally group 1 [NiFe] hydrogenases) serve to trace gas. In this work, we demonstrate, by using genetic disse- recycle the relatively high levels of H2 produced by biological ction and sensitive GC measurements, that the soil actinomycete processes (8, 13, 14). However, it has recently been shown that 2 Mycobacterium smegmatis mc 155 constitutively oxidizes subtro- certain soil-dwelling actinomycetes are capable of scavenging H2 pospheric concentrations of H2. We show that two membrane- at tropospheric concentrations (12, 15). -

The Impact of Nitrite on Aerobic Growth of Paracoccus Denitrificans PD1222
Katherine Hartop | January 2014 The Impact of Nitrite on Aerobic Growth of Paracoccus denitrificans PD1222 Submitted for approval by Katherine Rachel Hartop BSc (Hons) For the qualification of Doctor of Philosophy University of East Anglia School of Biological Sciences January 2014 This copy of the thesis has been supplied on conditions that anyone who consults it is understood to recognise that its copyright rests with the author and that use of any information derived there from must be in accordance with current UK Copyright Law. In addition, any quotation or extract must include full attribution. i Katherine Hartop | January 2014 Acknowledgments Utmost thanks go to my supervisors Professor David Richardson, Dr Andy Gates and Dr Tom Clarke for their continual support and boundless knowledge. I am also delighted to have been supported by the funding of the University of East Anglia for the length of my doctorial research. Thank you to the Richardson laboratory as well as those I have encountered and had the honour of collaborating with from the School of Biological Sciences. Thank you to Georgios Giannopoulos for your contribution to my research and support during the writing of this thesis. Thanks to my friends for their patience, care and editorial input. Deepest thanks to Dr Rosa María Martínez-Espinosa and Dr Gary Rowley for examining me and my research. This work is dedicated to my parents, Keith and Gill, and my family. ii Katherine Hartop | January 2014 Abstract The effect of nitrite stress induced in Paracoccus denitrificans PD1222 was examined using additions of sodium nitrite to an aerobic bacterial culture. -

1 Two Pathways for Thiosulfate Oxidation in The
bioRxiv preprint doi: https://doi.org/10.1101/683490; this version posted June 27, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Two pathways for thiosulfate oxidation in the alphaproteobacterial chemolithotroph Paracoccus thiocyanatus SST Moidu Jameela Rameez1, Prosenjit Pyne1,$, Subhrangshu Mandal1, Sumit Chatterjee1, Masrure 5 Alam1,#, Sabyasachi Bhattacharya1, Nibendu Mondal1, Jagannath Sarkar1 and Wriddhiman Ghosh1* Addresses: Department of Microbiology, Bose Institute, P-1/12 CIT Scheme VIIM, Kolkata 700054, India. 10 Present address: $National Institute of Cholera and Enteric Diseases, P-33, C. I. T Road, Scheme XM, Beliaghata, Kolkata 700 010, India. #Department of Biological Sciences, Aliah University, IIA/27, New Town, Kolkata-700160, India. *Correspondence: [email protected] 15 Running title: Thiosulfate oxidation via tetrathionate in an alphaproteobacterium Keywords: sulfur-chemolithotrophy, Sox multienzyme system, Alphaproteobacteria, thiosulfate oxidation via tetrathionate-intermediate, thiosulfate 20 dehydrogenase, tetrathionate oxidation Abstract Chemolithotrophic bacteria oxidize various sulfur species for energy and electrons, thereby 25 operationalizing biogeochemical sulfur cycles in nature. The best-studied pathway of bacterial sulfur-chemolithotrophy, involving direct oxidation of thiosulfate to sulfate (without any free intermediate) by the SoxXAYZBCD multienzyme system, is apparently the exclusive -

Characterization of the Virome of Paracoccus Spp. (Alphaproteobacteria) by Combined in Silico and in Vivo Approaches
www.nature.com/scientificreports OPEN Characterization of the virome of Paracoccus spp. (Alphaproteobacteria) by combined Received: 11 February 2019 Accepted: 17 May 2019 in silico and in vivo approaches Published: xx xx xxxx Przemyslaw Decewicz 1, Lukasz Dziewit 1, Piotr Golec 1, Patrycja Kozlowska2, Dariusz Bartosik1 & Monika Radlinska2 Bacteria of the genus Paracoccus inhabit various pristine and anthropologically-shaped environments. Many Paracoccus spp. have biotechnological value and several are opportunistic human pathogens. Despite extensive knowledge of their metabolic potential and genome architecture, little is known about viruses of Paracoccus spp. So far, only three active phages infecting these bacteria have been identifed. In this study, 16 Paracoccus strains were screened for the presence of active temperate phages, which resulted in the identifcation of fve novel viruses. Mitomycin C-induced prophages were isolated, visualized and their genomes sequenced and thoroughly analyzed, including functional validation of their toxin-antitoxin systems. This led to the identifcation of the frst active Myoviridae phage in Paracoccus spp. and four novel Siphoviridae phages. In addition, another 53 prophages were distinguished in silico within genomic sequences of Paracoccus spp. available in public databases. Thus, the Paracoccus virome was defned as being composed of 66 (pro)phages. Comparative analyses revealed the diversity and mosaicism of the (pro)phage genomes. Moreover, similarity networking analysis highlighted the uniqueness of Paracoccus (pro)phages among known bacterial viruses. Paracoccus spp. (Alphaproteobacteria) are metabolically versatile bacteria, that have been isolated from a wide range of environments in various geographical locations, e.g.: bioflters for the treatment of waste gases from an animal rendering plant in Germany (P. -

Comparative Genomics Analyses Indicate Differential Methylated Amine Utilization Trait Within Members of the Genus Gemmobacter
Environmental Microbiology Reports (2021) 13(2), 195–208 doi:10.1111/1758-2229.12927 Brief Report Comparative genomics analyses indicate differential methylated amine utilization trait within members of the genus Gemmobacter Eileen Kröber,1†* Mark R. Cunningham,2† while others (G. megaterium and G. nectariphilus) Julianna Peixoto,3 Lewis Spurgin,4 Daniela Wischer,4 have not. Ricardo Kruger 3 and Deepak Kumaresan 2* 1 Department of Symbiosis, Max-Planck Institute for Introduction Marine Microbiology, Bremen, Germany. 2School of Biological Sciences, Institute for Global Food Methylated amines (MAs) are ubiquitous in the environ- Security, Queen’s University Belfast, Belfast, UK. ment with a variety of natural and anthropogenic sources 3Department of Cellular Biology, University of Brasília, including the oceans, vegetation, sediments and organic- Brasília, Brazil. rich soils, animal husbandry, food industry, pesticides, 4School of Environmental Sciences, University of East sewage and automobiles, to mention only a few (Schade Anglia, Norwich, UK. and Crutzen, 1995; Latypova et al., 2010; Ge et al., 2011). Methylated amines are also known to influ- ence Earth’s climate, via a series of complex biological Summary and chemical interactions (Carpenter et al., 2012). Some Methylated amines are ubiquitous in the environment of the most abundant methylated amines found in the and play a role in regulating the earth’s climate via a atmosphere are trimethylamine (TMA), dimethylamine set of complex biological and chemical reactions. (DMA) and monomethylamine (MMA) (Ge et al., 2011). Microbial degradation of these compounds is thought Microbial metabolism of methylated amines involves both to be a major sink. Recently we isolated a facultative aerobic and anaerobic microorganisms, for example methylotroph, Gemmobacter sp. -

Paracoccus Niistensis Sp Nov., Isolated from Forest Soil, India
Author version: Antonie van Leeuwenhoek, vol.99(3); 2011; 501-506 Paracoccus niistensis sp nov., isolated from forest soil, India Syed G.Dastager1,2, Deepa C.K2, Wen-Jun Li3, Shu-Kun Tang3 and Ashok Pandey2 Present Address: 2Biological Oceanography Division, National Institute of Oceanography (CSIR), Dona Paula-403004, Goa, India 1National Institute for Interdisciplinary Science and technology (CSIR), Trivandrum-695019, India. 3Laboratory for Conservation and Utilization of Bio-Resources, Yunnan Institute of Microbiology, Yunnan University, Kunming, Yunnan 650091, People’s Republic of China Author for correspondence: Dr.Syed G Dastager Tel: 832-2450446, 447; Fax: +91-832- 2450606 Email: [email protected], [email protected], Running title: Paracoccus niistensis sp.nov. Abstract A Gram-negative, non-motile, catalase-positive and oxidase-positive, aerobic bacterium designated as NII-0918T was isolated from soil sample in Western ghat forest, India. 16S rRNA gene sequence analysis showed that strain NII-0918T was belongs to the subclass α-Proteobacteria, being related to genus Paracoccus, and sharing highest sequence similarity with Paracoccus chinensis NBRC 104937T(99.4%), Paracoccus marinus NBRC 100640T (97.3%), Paracoccus koreensis Ch05T (97.1%) and Paracoccus kondratievae GBT(97.0%). Other members of Paracoccus showing below 97.0% similarity. The DNA–DNA hybridization values between these four strains and NII-0918T were 44.7, 28, T 32 and 41% respectively. The major fatty acids of strain NII-0918 were summed feature 7 (C18:1 ω7c/ ω 9t/ ω 12t) (83.0 %) and C18:0 (12.5 %). Ubiquinone Q-10 was detected as the major respiratory quinone. The G+C content of genomic DNA of NII-0918T was 66.6 mol%. -
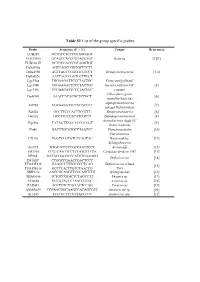
Table S1 List of the Group Specific Probes
Table S1 List of the group specific probes. Probe Sequence (5’ – 3’) Target References EUB338 GCTGCCTCCCGTAGGAGT EUB338 II GCAGCCACCCGTAGGTGT Bacteria [1][2] EUB338 III GCTGCCACCCGTAGGTGT Delta495a AGTTAGCCGGTGCTTCTT Delta495b AGTTAGCCGGCGCTTCCT Deltaproteobacteria [3,4] Delta495c AATTAGCCGGTGCTTCCT Lgc354a TGGAAGATTCCCTACTGC Firmicutes (Gram+ Lgc354b CGGAAGATTCCCTACTGC bacteria with low GC [5] Lgc354c CCGAAGATTCCCTACTGC content) Chloroflexi (green Gnsb941 AAACCACACGCTCCGCT [6] nonsulfur bacteria) Alphaproteobacteria Alf968 GGTAAGGTTCTGCGCGTT [7] (except Rickettsiales) Bet42a GCCTTCCCACTTCGTTT Betaproteobacteria [8] Gam2a GCCTTCCCACATCGTTT Gammaproteobacteria [8] Actinobacteria (high GC Hgc69a TATAGTTACCACCGCCGT [9] Gram+ bacteria) Pla46 GACTTGCATGCCTAATCC Planctomycetales [10] Flavobacteria, Cf319a TGGTCCGTGTCTCAGTAC Bacteroidetes, [11] Sphingobacteria Arc915 GTGCTCCCCCGCCAATTCCT Archaea [12] TM7905 CCGTCAATTCCTTTATGTTTTA Candidate division TM7 [13] DF988* GATACGACGCCCATGTCAAGGG Defluvicoccus [14] DF1020* CCGGCCGAACCGACTCCC TFO-DF218 GAAGCCTTTGCCCCTCAG Defluvicoccus related [15] TFO-DF618 GCCTCACTTGTCTAACCG TFO SBR9-1a AAGCGCAAGTTCCCAGGTTG Sphingomonas [16] THAU646 TCTGCCGTACTCTAGCCTT Thauera sp. [17] AZO644 GCCGTACTCTAGCCGTGC Azoarcus sp. [18] PAR651 ACCTCTCTCGAACTCCAG Paracoccus [19] AMAR839 CCGAACGGCAAGCCACAGCGTC Amaricoccus sp. [20] ACI145 TTTCGCTTCGTTATCCCC Acidovorax spp. [21] Table S2 Primers used in PCR and Sequencing. Primers Sequence (5’ – 3’) PCR 27f AGAGTTTGATCMTGGCTCAG 1492r TACGGYTACCTTGTTACGACTT T7f TAATACGACTCACTATAGGG -

EPA Advice on Application APP201895 – Determination on the New Organism Status of Pseudomonas Monteilii, Rhodococcus Pyridiniv
Staff Assessment Report EPA advice on application APP201895 – determination on the new organism status of Pseudomonas monteilii, Rhodococcus pyridinivorans, Paracoccus pantotrophus, and Nitrosomonas eutropha June 2014 2 EPA advice for application APP201895 Executive summary and recommendation Application APP201895, submitted by Neil Pritchard of NPN Ltd., Napier, seeks a determination on the new organism status of four bacterial species (Pseudomonas monteilii, Rhodococcus pyridinivorans, Paracoccus pantotrophus and Nitrosomonas eutropha). After reviewing the information, EPA staff recommend that the Hazardous Substances and New Organisms (HSNO) Decision Making Committee determines that Pseudomonas monteilii, Rhodococcus pyridinivorans, Paracoccus pantotrophus and Nitrosomonas eutropha are not new organisms for the purposes of the HSNO Act. However, should new evidence be found regarding the new organism status of any of these organisms, new determinations can be sought. July 2014 3 EPA advice for application APP201895 Table of Contents Executive summary and recommendation ............................................................................................ 2 Table of Contents .................................................................................................................................. 3 1. Introduction .................................................................................................................................. 4 2. Organism description..................................................................................................................