SEA-MAL-263 A4 Cover
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
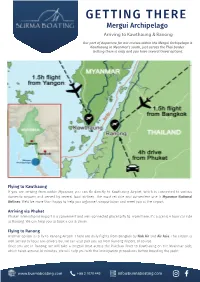
GETTING THERE Mergui Archipelago Arriving to Kawthaung & Ranong
GETTING THERE Mergui Archipelago Arriving to Kawthaung & Ranong Our port of departure for our cruises within the Mergui Archipelago is Kawthaung in Myanmar’s south, just across the Thai border. Getting there is easy and you have several travel options. Flying to Kawthaung If you are arriving from within Myanmar, you can fly directly to Kawthaung Airport, which is connected to various domestic airports and served by several local airlines. The most reliable and convenient one is Myanmar National Airlines. We’d be more than happy to help you organise transportation and meet you at the airport. Arriving via Phuket Phuket International Airport is a convenient and well-connected place to fly to. From there, it’s a scenic 4 hour car ride to Ranong. We can help you to book a car & driver. Flying to Ranong Another option is to fly to Ranong Airport. There are daily flights from Bangkok by Nok Air and Air Asia. The airport is well served by local taxi drivers but we can also pick you up from Ranong Airport, of course. Once you are in Ranong, we will take a longtail boat across the Pakchan River to Kawthaung on the Myanmar side, which takes around 30 minutes. We will help you with the immigration procedures before boarding the yacht. www.burmaboating.com +66 2 1070 445 [email protected] GETTING THERE Mergui Archipelago Crossing the Thai-Myanmar border In the case you arrive through the Thailand side and doesn’t want our assistance, here is a quick step by step instruction to cross the border between the 2 countries. -

Military Brotherhood Between Thailand and Myanmar: from Ruling to Governing the Borderlands
1 Military Brotherhood between Thailand and Myanmar: From Ruling to Governing the Borderlands Naruemon Thabchumphon, Carl Middleton, Zaw Aung, Surada Chundasutathanakul, and Fransiskus Adrian Tarmedi1, 2 Paper presented at the 4th Conference of the Asian Borderlands Research Network conference “Activated Borders: Re-openings, Ruptures and Relationships”, 8-10 December 2014 Southeast Asia Research Centre, City University of Hong Kong 1. Introduction Signaling a new phase of cooperation between Thailand and Myanmar, on 9 October 2014, Thailand’s new Prime Minister, General Prayuth Chan-o-cha took a two-day trip to Myanmar where he met with high-ranked officials in the capital Nay Pi Taw, including President Thein Sein. That this was Prime Minister Prayuth’s first overseas visit since becoming Prime Minister underscored the significance of Thailand’s relationship with Myanmar. During their meeting, Prime Minister Prayuth and President Thein Sein agreed to better regulate border areas and deepen their cooperation on border related issues, including on illicit drugs, formal and illegal migrant labor, including how to more efficiently regulate labor and make Myanmar migrant registration processes more efficient in Thailand, human trafficking, and plans to develop economic zones along border areas – for example, in Mae 3 Sot district of Tak province - to boost trade, investment and create jobs in the areas . With a stated goal of facilitating border trade, 3 pairs of adjacent provinces were named as “sister provinces” under Memorandums of Understanding between Myanmar and Thailand signed by the respective Provincial governors during the trip.4 Sharing more than 2000 kilometer of border, both leaders reportedly understood these issues as “partnership matters for security and development” (Bangkok Post, 2014). -

Transport Logistics
MYANMAR TRADE FACILITATION THROUGH LOGISTCS CONNECTIVITY HLA HLA YEE BITEC , BANGKOK 4.9.15 [email protected] Total land area 677,000sq km Total length (South to North) 2,100km (East to West) 925km Total land boundaries 5,867km China 2,185km Lao 235km Thailand 1,800km Bangladesh 193km India 1,463km Total length of coastline 2,228km Capital : Naypyitaw Language :Myanmar MYANMAR IN 2015 REFORM & FAST ECONOMIC DEVELOPMENTS SIGNIFICANT POTENTIAL CREDIBILITY AMONG ASEAN NATIONS GATE WAY “ CHINA & INDIA & ASEAN” MAXIMIZING MULTIMODALTRANSPORT LINKAGES EXPEND GMS ECONOMIC TRANSPORT CORRIDORS EFFECTIVE EXTENSION INTO MYANMAR INTERNATIONAL INSTITUTION TRADE AND LOTISGICS SUPPLY CHAIN TRANSPARENCY & PREDICTABILITY LEGAL & REGULATORY FREAMEWORK INFRASTUCTURE INFORMATION CORRUPTION FIANACIAL SERVICE “STRENGTHEING SME LOGISTICS” INDUSTRIAL ZONE DEVELOPMENT 7 NEW IZ KYAUk PHYU Yadanarbon(MDY) SEZ Tart Kon (NPD) Nan oon Pa han 18 Myawadi Three pagoda Existing IZ Pon nar island Yangon(4) Mandalay Meikthilar Myingyan Yenangyaing THI LA WAR Pakokku SEZ Monywa Pyay Pathein DAWEI Myangmya SEZ Hinthada Mawlamyaing Myeik Taunggyi Kalay INDUSTRIES CATEGORIES Competitive Industries Potential Industries Basic Industries Food and Beverages Automobile Parts Agricultural Machinery Garment & Textile Industrial Materials Agricultural Fertilizer Household Woodwork Minerals & Crude Oil Machinery & spare parts Gems & Jewelry Pharmaceutical Electrical & Electronics Construction Materials Paper & Publishing Renewable Energy Household products TRANSPORT -

Preparatory Survey for the Project for Strengthening Connectivity of International Highway in Mekong Region Final Report Phase-I
MINISTRY OF CONSTRUCTION REPUBLIC OF THE UNION OF MYANMAR PREPARATORY SURVEY FOR THE PROJECT FOR STRENGTHENING CONNECTIVITY OF INTERNATIONAL HIGHWAY IN MEKONG REGION FINAL REPORT PHASE-I SURVEY REPORT (FEASIBILITY STUDY) DECEMBER 2016 JAPAN INTERNATIONAL COOPERATION AGENCY ORIENTAL CONSULTANTS GLOBAL CO., LTD. INTERNATIONAL DEVELOPMENT CENTER OF JAPAN CTI ENGINEERING CO., LTD. 1R NIPPON KOEI CO., LTD. CR(4) 16-077 MINISTRY OF CONSTRUCTION REPUBLIC OF THE UNION OF MYANMAR PREPARATORY SURVEY FOR THE PROJECT FOR STRENGTHENING CONNECTIVITY OF INTERNATIONAL HIGHWAY IN MEKONG REGION FINAL REPORT PHASE-I SURVEY REPORT (FEASIBILITY STUDY) DECEMBER 2016 JAPAN INTERNATIONAL COOPERATION AGENCY ORIENTAL CONSULTANTS GLOBAL CO., LTD. INTERNATIONAL DEVELOPMENT CENTER OF JAPAN CTI ENGINEERING CO., LTD. NIPPON KOEI CO., LTD. Currency Equivalents USD 1.00 = MMK 1030.9 = JPY 120.4 (May 2015) *MMK: Myanmar Kyat Republic of the Union of Myanmar Location map of the survey area India China Bangladesh Laos Myanmar Legend Thailand Target Route GMS East-West Economic Corridor GMS Southern Economic Corridor Asian Highway Route Major city Basic data of Myanmar Source: MOFA ■Area Approximately 680,000km2 ■Total trade ■Population Approximately 51.41 million (Central Statistical Organization (FY2013/14)) (Sep, 2014 (Provisional statistics by (1)Export:Approximately 11.2 billion USD Ministry of Immigration and Population, (2)Import:Approximately 13.7 billion USD Myanmar)) ■Major trading items ■Capital Nay Pyi Taw (1)Export:Natural gas, beans, clothing, teak, etc. (2)Import:Machinery, essential oil, manufactured ) ■Ethnicity Burmese (Approximately 70% and goods, etc. Many other ethnic minorities ■Currency Kyat 1USD= 1,285Kyat (Rate of central ■Language Burmese bank of Myanmar: Feb 2016) ■Religion Buddhism (Approximately 90%), ■Japanese ODA Christian, Muslim etc. -

Covid-19 Response Situation Report 7 | 30 May 2020
IOM MYANMAR COVID-19 RESPONSE SITUATION REPORT 7 | 30 MAY 2020 7,181 migrants returned from Thailand from 22 to 28 May, mainly from Myawaddy-Mae Sot 2,848 migrants returned from China from 22 to 28 May, through Nan Taw and Chin Shwe Haw A COVID-19 risk communication session at Shwe Myawaddy Quarantine Centre in Myawaddy, Kayin State. © IOM 2020 SITUATION OVERVIEW Returns from Thailand began picking up this week, and from Government during the process of applying for employment 22 to 28 May, 7,031 migrants returned through Myawaddy- cards. PRAs are also required to communicate these regulations Mae Sot, and 150 returned through Kawthaung-Ranoung. to respective Thai employers. Should PRAs not follow these These include 1,979 migrants whose return was facilitated instructions, DOL will revoke the license of the PRA concerned. following coordination between the Embassy of Myanmar in Thailand and Thai authorities, with the rest self-arranging their return. Returnees were also tested for COVID-19 upon arrival to Myanmar, with most returnees, upon confirmation of negative test results, being transported to their communities of origin for quarantine. A total of 45,168 migrants returned from Thailand from 22 March to 28 May. The Department of Labour (DOL) issued a letter on 22 May to the Myanmar Overseas Employment Agency Federation (MOEAF) on the restarting of recruitment procedures for Myanmar migrants seeking migration and employment in Thailand. The letter announced that recruitment procedures are on hold until 31 May, and that Thai authorities will accept migrant workers who have health certificates and who undergo Latrines provided by IOM at a quarantine facility in Myawaddy, Kayin State. -

Status of Transport Connectivity Including Transit Facilitation In
New Delhi, India 19-20 November 2014 Aye Aye Hla,Director Ministry of Rail Transportation Myanmar Myanmar Profile National data Population - 52 millions Land Area - 676,578 sq.km Arable - 19.2% Coastal Line - 2800 km Road Length - 148690 km ESCAP Membership - 19 April 1948 Myanmar is a member of such sub-regional networks as the India- Myanmar-Thailand Highway, the ASEAN Mekong Basin Development Cooperation (AMBDC), the Bangladesh-China-India-Myanmar (BCIM) Economic Corridor, the Mekong-Ginga Cooperation and the Mekong Sub- region. Myanmar Perspective Transport is a key to sustainable development and regional integration. Transport connectivity contributes towards the regional development, economic growth and fortifies relations among the neighbouring countries. Myanmar believes a stronger integrated partnership in the Asia Pacific region will greatly contribute to member countries and people as a whole. The development of the infrastructural network will not only expand the region market but also capture international markets. It plays the key role for the country’s economic growth and regional connectivity, integration and mutual benefits. Nowadays, most countries in the Asia have been connected through the Asian Highway Network and the Trans-Asian Railway Network, which will benefit coastal countries and small island developing states by means of maritime services. Myanmar Perspective Myanmar is in a geographically strategic position to greatly benefit both South Asia and Southeast Asia. Myanmar is now regarded as a newly emerging destination for business on account of its strategic geographical location, moderate climate fit for the cultivation of various crops, huge market size, and high production output at low costs. -

Inside Cover
Acknowledgements The Improving ATS Data and Information Systems project is managed by the United Nations Office on Drugs and Crime Regional Centre for East Asia and the Pacific. It is the primary mechanism in the region for monitoring drug abuse patterns and trends and for disseminating the most up-to-date information on those patterns and trends. Grateful appreciation is expressed to the national drug control agencies participating in the Drug Abuse Information Network for Asia and the Pacific (DAINAP) in Australia, Brunei Darussalam, Cambodia, China, Indonesia, Japan, Lao PDR, Malaysia, Myanmar, Philippines, Singapore, Thailand and Viet Nam and to the staff of each agency and affiliated office for compiling and submitting the data requested and for reviewing and clarifying their data submissions prior to publication of the report. Without the significant effort of the 13 national coordinators and drug control agencies participating in the DAINAP surveillance project, this report could not have been compiled. Particular acknowledgement is given to the Governments of Australia and Japan for providing funding to support the development and maintenance of this activity. The core team that prepared this report was comprised of Nicholas J. Kozel, Expert Consultant, Johannes Lund, Data Integrity Consultant, Jeremy Douglas, UNODC Regional Project Coordinator for Improving ATS Data and Information Systems (TDRASF97) and Rebecca McKetin, Senior Research Fellow National Drug and Alcohol Research Centre. For more information about the Improving -
Tourist Attractions in Tanintharyi
Huai Ma lwe Thungyai - Huai Kha daung Khaeng Wildlife Kha U t h a i 634m Thungyai Sanctuaries Khaeng Naresuan Phu Toei T h a n i C h a i Khuen Si Nakarin N a t 15°0'0"N Khao ^_ selected tourism attraction Tourist attractions inurÇmvSnfhc&D;onfrsm; Tanintharyi pdwf0ifpm;onfhae&mr Laem dive site Mon State current and selectedKhlong potential attractions for tourisma&ikyfEdkifonfhae&m (DoHT and other sources) Lamngu S u p h a n Tanintharyi Region Admin. boundaryS u p h a n Settlements Tourist attractions/sites tkyfcsKyfrIe,fedrdwfrsm;B u r i vlaexdkif&ma'orsm; ^¬ island tourist attraction international ^¬ region capital tjynfjynfqdkif&mEdkifiHe,fedrdwf wdkif;a'oBuD;^NrdKU ^¬ district capital other tourist attraction State/Region ^¬ DoHT Zin ba c½dkif^NrdKU Taung data from wdkif;ESihfjynfe,fe,fedrdwf 464m Thong ^_ planned CBT township capital District / Changwat Pha Phum Chalearm NrdKUe,f^NrdKU Dawei !. other (and potential) tourist attraction Rattanakosin c½dkife,fedrdwf sub-township capital Kaleinaung Colonial old town, broom, mats & cashew factory, “9 Pagodas”, Township NrdKUe,fcGJ^NrdKU Tanintharyi Cultural Museum, MyoMarket, Malingapi, Ma Saw Po dive sites NrdKUe,fe,fedrdwf & Saw Si Gong Chinese Temple, Ancient cities (Wedi, Thargara, ..), other town Sandaw NrdKU Mosques, Hindu Temples, ... Shin ?! Kanchanaburi mangrove / 'Da&awm Paleekari Hotel, Garden Hotel, Hotel Zeyar Thet San Pagoda Tanintharyi Tourist checkpoint (planned) !( village tract main village Hotel Shwe Maung Than, Maung Lay Hotel, Golden Guest Hotel, Nature Reserve mudflat -

Thailand Burma China Laos
Burmese border refugee sites with population figures: February 2007 1 TBBC 2 Feb-07 +/(-) Female Male Total Jan-07 CHINA Chiengmai Province Shan Keng Tung WH Wieng Heng (Shan Refugees) 317 287 604 - State Taunggyi Mae Hong Son Province Site 1 Ban Kwai/Nai Soi 9,479 10,260 19,739 66 BURMA Tachilek MaeFaLuang LAOS Site 2 Ban Mae Surin 1,767 1,890 3,657 25 MongYawn Naypyidaw Fang Mae Sai K1 Mae La Oon (Site 3) 7,456 8,248 15,704 (115) Loikaw PaMaPhaDoiDa Karenni Site 1 WH K2 Mae Ra Ma Luang (Site 4) 7,817 8,128 15,945 329 Toungoo State Mae Hong Son Subtotal: 26,519 28,526 55,045 305 Karen State Site 2 Chiang Mai Tak Province K3 Mae La 24,180 25,296 49,476 113 Pegu Mae Sariang Division K4 Umpiem Mai 9,440 10,161 19,601 18 Papun THAILAND Mon K1&2 K5 Nu Po 7,421 7,979 15,400 70 State Manerplaw K3Tha Song Yang Subtotal: 41,041 43,436 84,477 201 Rangoon Mae Ramat Kanchanaburi Province Pa-an Mae Sot Myawaddy Phetchabun K6 Ban Don Yang 2,323 2,268 4,591 (75) K4 Moulmein Ratchaburi Province Umphang K5 K7 Tham Hin 4,794 5,056 9,850 168 Three Pagodas Pass Total for sites in Thailand: 74,994 79,573 154,567 599 M1 Ye K6 M2 Sangklaburi M3 M4 State of Origin of Registered Population 62% Karen 5% Pegu Kanchanaburi Bangkok Tavoy 13% Karenni 4% Unknown K7 Ratchaburi 9% Tenasserim 2% Other (Chin, Kachin, Irrawaddy, Magwe, Mandalay, Tenasserim Suan Phung 5% Mon Rakhine, Rangoon, Sagaing, Shan) Division A N D A M A N S E A IDP Site Wieng Heng: Camp Committee I N D I A Mergui C H I N A PAKISTAN Prachuap Khiri Khan Sites 1 & 2: Karenni Refugee Committee (KnRC) -

Myanmar National Transport Master Plan; MYT-Plan
Overview of Transport Operational Connectivity, Challenges for Integrated Transport, Challenges to Seamless Border Crossing and National Strategies for Transport Facilitation Workshop on Strengthening Transport Operational Connectivity among CLMV-T Phnom Penh, Cambodia 25 September 2017 Aye Aye Hla Joint Secretary of NTFC Ministry of Transport and Communications Myanmar Contents ➢ Introduction ➢ Overview of transport operational connectivity • Current status of cross-border transport facilitation of International Transport Agreements including implementation • Regional Connectivity ➢ National Strategies (Plans) for Transport Development ➢ Current use of SWI and SSI at the border crossings ➢ Challenges to transport facilitation including customs formalities Introduction National data Population - 52 millions Land Area - 676,578 sq.km Arable - 19.2% Coastal Line - 2800 km Road Length - 148690 km ESCAP Membership - 19 April 1948 And also Myanmar is a member of such sub-regional networks as ASEAN, Greater Mekong Sub-region (GMS), BIMSTEC, the ASEAN Mekong Basin Development Cooperation (AMBDC), the Bangladesh-China-India- Myanmar (BCIM) Economic Corridor and the Mekong-Ginga Cooperation (MGC). And also involved in the Tran-Asian Railways and Tran-Asian Highways networks. • Myanmar is in a geographically strategic position and has been identified as an important transport link to South and Southeast Asia. • Myanmar is now regarded as a newly emerging destination for business on account of its strategic geographical location, moderate climate fit for the cultivation of various crops, huge market size, and high production output at low costs. • Myanmar has recognized the importance of improving its domestic transport network, including roads that connect to the sub-regional corridors. Our country also has an extensive rail network, an important deep sea ports, and a domestic river transport network. -

Covid-19 Response Situation Report 11 | 6 August 2020
IOM MYANMAR COVID-19 RESPONSE SITUATION REPORT 11 | 6 AUGUST 2020 10,893 migrants returned from Thailand through border checkpoints from 17 July to 5 August 141,710 migrants returned through border checkpoints to date Migrant Resource Centre (MRC) Counsellor, in coordination with the Department of Labour (DOL), distributing humanitarian assistance to returning migrants in Myawaddy, Kayin State. © IOM 2020 SITUATION OVERVIEW Returns from Thailand continued at a steady pace on the Thailand due to travel restrictions. On 4 August, a Cabinet second half of July and the first week of August, with 10,893 Resolution approved measures for the extension of stay and re- (4,520 female, 6,373 male) returns from 17 July to 5 August. A employment of MOU migrants already in Thailand. But the further 949 Myanmar nationals returned via Myanmar deployment of new MOU migrants still awaits Cabinet Government Assisted Relief flights between 17 to 26 July, approval, and procedures and costs are still being discussed. including from Hong Kong, Singapore, Korea, Australia and The Myanmar Embassy in Thailand is collecting the advance Jordan, among others. Since the middle of March, a total of voting list of Myanmar nationals in Thailand, including of MOU 141,710 migrants have returned to Myanmar via land border migrants, in preparation for the upcoming elections. checkpoints from Thailand (97,342), China (44,051) and Lao PDR (317), with an additional 9,492 Myanmar nationals returning via relief flights. The Myanmar Government extended COVID-19 related restrictions until 15 August. This is the fourth extension and it retains the nationwide 00:00 AM to 4:00 AM curfew, the ban on international commercial flights into Myanmar, the temporary suspension of visas on arrival and e-visas, and the temporary suspensions on overseas employment processes (including MOU recruitment and deployment). -

Rapid Urban Diagnostic Report
THE REPUBLIC OF THE UNION OF MYANMAR RAPID URBAN DIAGNOSTIC REPORT Prepared for Department of Urban and Housing Development, Ministry of Construction With technical assistance from UN Habitat RAPID URBAN DIAGNOSTIC REPORT MYANMAR TABLE OF CONTENTS A. CONTEXT AND BACKGROUND OF RAPID URBAN DIAGNOSTIC REPORT MYANMAR P. 4 A.I EXECUTIVE SUMMARY P. 4 A.II GENERAL BACKGROUND P. 5 A.III BACKGROUND OF RAPID URBAN DIAGNOSTIC P. 5 A.IV PURPOSE OF RAPID URBAN DIAGNOSTIC REPORT MYANMAR P. 6 B. RAPID URBAN DIAGNOSTIC REPORT MYANMAR P. 7 B.I URBANIZATION P. 7 B.I.1 CURRENT LEVEL OF URBANIZATION P. 7 B.I.2 URBANIZATION IN MYANMAR IN REGIONAL PERSPECTIVE P. 9 B.I.3 DRIVERS AND TRENDS OF URBANIZATION P. 11 B.I.3.1 DRIVERS OF URBANIZATION P. 11 B.I.3.2 PROJECTION OF URBANIZATION P. 12 B.I.3.3 SPATIAL DIMENSION OF URBANIZATION IN MYANMAR P. 13 B.II MYANMAR’S SYSTEM OF URBAN CENTERS AND GROWTH CORRIDORS P. 14 B.III URBAN GOVERNANCE IN TOWNSHIPS/SECONDARY CITIES P. 19 B.III.1 CONSTITUTIONAL FRAMEWORK P. 19 B.III.2 ADMINISTRATIVE ORGANIZATION AT THE TOWNSHIP LEVEL P. 20 B.III.2.1 THE TOWNSHIP ADMINISTRATOR AS CENTRAL ACTOR AT TOWNSHIP LEVEL P. 20 B.III.2.2 THE TOWNSHIP COMMITTEES P. 20 B.III.2.3 A SPECIAL CASE: THE TOWNSHIP MUNICIPAL AFFAIRS COMMITTEE AND THE DEPARTMENT OF MUNICIPAL AFFAIRS (DEVELOPMENT AFFAIRS ORGANIZATION) P. 22 B.III.2.3.1 ORIGINS OF DMA P. 22 B.III.2.3.2 DMA’S FISCAL POSITION P.