Veeramani A, Et Al. Demography, Activity Pattern, Food and Feeding Habits of Primates in Nilgiris, Tamil Nadu, India. Int J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Taxonomy of Primates in the Laboratory Context
P0800261_01 7/14/05 8:00 AM Page 3 C HAPTER 1 The Taxonomy of Primates T HE T in the Laboratory Context AXONOMY OF P Colin Groves RIMATES School of Archaeology and Anthropology, Australian National University, Canberra, ACT 0200, Australia 3 What are species? D Taxonomy: EFINITION OF THE The biological Organizing nature species concept Taxonomy means classifying organisms. It is nowadays commonly used as a synonym for systematics, though Disagreement as to what precisely constitutes a species P strictly speaking systematics is a much broader sphere is to be expected, given that the concept serves so many RIMATE of interest – interrelationships, and biodiversity. At the functions (Vane-Wright, 1992). We may be interested basis of taxonomy lies that much-debated concept, the in classification as such, or in the evolutionary implica- species. tions of species; in the theory of species, or in simply M ODEL Because there is so much misunderstanding about how to recognize them; or in their reproductive, phys- what a species is, it is necessary to give some space to iological, or husbandry status. discussion of the concept. The importance of what we Most non-specialists probably have some vague mean by the word “species” goes way beyond taxonomy idea that species are defined by not interbreeding with as such: it affects such diverse fields as genetics, biogeog- each other; usually, that hybrids between different species raphy, population biology, ecology, ethology, and bio- are sterile, or that they are incapable of hybridizing at diversity; in an era in which threats to the natural all. Such an impression ultimately derives from the def- world and its biodiversity are accelerating, it affects inition by Mayr (1940), whereby species are “groups of conservation strategies (Rojas, 1992). -
Telephone Numbers
DISTRICT DISASTER MANAGEMENT AUTHORITY THANJAVUR IMPORTANT TELEPHONE NUMBERS DISTRICT EMERGENCY OPERATION CENTRE THANJAVUR DISTRICT YEAR-2018 2 INDEX S. No. Department Page No. 1 State Disaster Management Department, Chennai 1 2. Emergency Toll free Telephone Numbers 1 3. Indian Meteorological Research Centre 2 4. National Disaster Rescue Team, Arakonam 2 5. Aavin 2 6. Telephone Operator, District Collectorate 2 7. Office,ThanjavurRevenue Department 3 8. PWD ( Buildings and Maintenance) 5 9. Cooperative Department 5 10. Treasury Department 7 11. Police Department 10 12. Fire & Rescue Department 13 13. District Rural Development 14 14. Panchayat 17 15. Town Panchayat 18 16. Public Works Department 19 17. Highways Department 25 18. Agriculture Department 26 19. Animal Husbandry Department 28 20. Tamilnadu Civil Supplies Corporation 29 21. Education Department 29 22. Health and Medical Department 31 23. TNSTC 33 24. TNEB 34 25. Fisheries 35 26. Forest Department 38 27. TWAD 38 28. Horticulture 39 29. Statisticts 40 30. NGO’s 40 31. First Responders for Vulnerable Areas 44 1 Telephone Number Officer’s Details Office Telephone & Mobile District Disaster Management Agency - Thanjavur Flood Control Room 1077 04362- 230121 State Disaster Management Agency – Chennai - 5 Additional Cheif Secretary & Commissioner 044-28523299 9445000444 of Revenue Administration, Chennai -5 044-28414513, Disaster Management, Chennai 044-1070 Control Room 044-28414512 Emergency Toll Free Numbers Disaster Rescue, 1077 District Collector Office, Thanjavur Child Line 1098 Police 100 Fire & Rescue Department 101 Medical Helpline 104 Ambulance 108 Women’s Helpline 1091 National Highways Emergency Help 1033 Old Age People Helpline 1253 Coastal Security 1718 Blood Bank 1910 Eye Donation 1919 Railway Helpline 1512 AIDS Helpline 1097 2 Meteorological Research Centre S. -

Kumbakonam Bye-Pass - Land Acquisition Sanctioned - Revised Administrative Sanction Accorded - Orders Issued
ABSTRACT Highways Department - Tamil Nadu Road Sector Project - Kumbakonam bye-pass - Land Acquisition sanctioned - Revised Administrative Sanction accorded - Orders Issued. HIGHWAYS (HN1) DEPARTMENT G.O. Ms. No. 189 Dated: 17-10-2002 Read the following 1. G.O. Ms. No. 533 TPT dated. 23.5.1989 2. G.O..Ms .No.59 Highways (HN1) Department dated 16.3.2001 3. From the Project Director ,Tamil Nadu Road Sector Project letter No.1414/2002 /B Section dated. 23.7.2002. ORDER: In the G.O. first read above the Government have accorded administrative sanction of Rs.40.00 lakhs for acquisition of lands for Kumbakonam bye-pass. 2. In the G.O. Second read above the Government have sanctioned Rs.49.50 Crores for acquisition of lands under Tamil Nadu Road Sector Project . 3. In his letter third read above the Project Director, Tamil Nadu Road Sector Project has stated that the approved Kumbakonam bye-pass alignment starts at km 2/10 of Kumbakonam-Sirkazhi road and ends at km. 129/4 of Kumbakonam-Thanjavur road for a length of 8.20 km. The estimate for Rs.44.00 lakhs was technically sanctioned by Chief Engineer (General). This envisages acquisition of lands of about 26 hectares in 10 villages. Lands were acquired to an extent of 11.05.00 hectares at a cost of Rs.34.58 lakhs in 6 villages. Lands were partly acquired in 4 other villages for an extent of 9.75.50 hectares. The value of these lands of 9.75.50 hectares is Rs.36.15 lakhs. -

Nonhuman Primates
Zoological Studies 42(1): 93-105 (2003) Dental Variation among Asian Colobines (Nonhuman Primates): Phylogenetic Similarities or Functional Correspondence? Ruliang Pan1,2,* and Charles Oxnard1 1School of Anatomy and Human Biology, University of Western Australia, Crawley, Perth, WA 6009, Australia 2Institute of Zoology, Chinese Academy of Sciences, Beijing 100080, China (Accepted August 27, 2002) Ruliang Pan and Charles Oxnard (2003) Dental variation among Asian colobines (nonhuman primates): phy- logenetic similarities or functional correspondence? Zoological Studies 42(1): 93-105. In order to reveal varia- tions among Asian colobines and to test whether the resemblance in dental structure among them is mainly associated with similarities in phylogeny or functional adaptation, teeth of 184 specimens from 15 Asian colobine species were measured and studied by performing bivariate (allometry) and multivariate (principal components) analyses. Results indicate that each tooth shows a significant close relationship with body size. Low negative and positive allometric scales for incisors and molars (M2s and M3s), respectively, are each con- sidered to be related to special dental modifications for folivorous preference of colobines. Sexual dimorphism in canine eruption reported by Harvati (2000) is further considered to be associated with differences in growth trajectories (allometric pattern) between the 2 sexes. The relationships among the 6 genera of Asian colobines found greatly differ from those proposed in other studies. Four groups were detected: 1) Rhinopithecus, 2) Semnopithecus, 3) Trachypithecus, and 4) Nasalis, Pygathrix, and Presbytis. These separations were mainly determined by differences in molar structure. Molar sizes of the former 2 groups are larger than those of the latter 2 groups. -
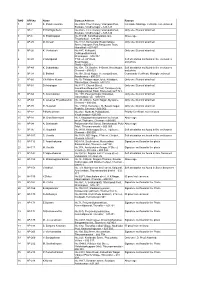
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -

Ranging Bonnet Macaques, Macaca Radiata
Flexibility in Food Extraction Techniques in Urban Free- Ranging Bonnet Macaques, Macaca radiata Madhur Mangalam1, Mewa Singh1,2* 1 Biopsychology Laboratory, University of Mysore, Mysore, India, 2 Evolutionary & Organismal Biology Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, India Abstract Non-human primate populations, other than responding appropriately to naturally occurring challenges, also need to cope with anthropogenic factors such as environmental pollution, resource depletion, and habitat destruction. Populations and individuals are likely to show considerable variations in food extraction abilities, with some populations and individuals more efficient than others at exploiting a set of resources. In this study, we examined among urban free-ranging bonnet macaques, Macaca radiata (a) local differences in food extraction abilities, (b) between-individual variation and within-individual consistency in problem-solving success and the underlying problem-solving characteristics, and (c) behavioral patterns associated with higher efficiency in food extraction. When presented with novel food extraction tasks, the urban macaques having more frequent exposure to novel physical objects in their surroundings, extracted food material from PET bottles and also solved another food extraction task (i.e., extracting an orange from a wire mesh box), more often than those living under more natural conditions. Adults solved the tasks more frequently than juveniles, and females more frequently than males. Both solution-technique and problem-solving characteristics varied across individuals but remained consistent within each individual across the successive presentations of PET bottles. The macaques that solved the tasks showed lesser within-individual variation in their food extraction behavior as compared to those that failed to solve the tasks. -

Macaques ( Macaca Leonina ): Impact on Their Seed Dispersal Effectiveness and Ecological Contribution in a Tropical Rainforest at Khao Yai National Park, Thailand
Faculté des Sciences Département de Biologie, Ecologie et Environnement Unité de Biologie du Comportement, Ethologie et Psychologie Animale Feeding and ranging behavior of northern pigtailed macaques ( Macaca leonina ): impact on their seed dispersal effectiveness and ecological contribution in a tropical rainforest at Khao Yai National Park, Thailand ~ ~ ~ Régime alimentaire et déplacements des macaques à queue de cochon ( Macaca leonina ) : impact sur leur efficacité dans la dispersion des graines et sur leur contribution écologique dans une forêt tropicale du parc national de Khao Yai, Thaïlande Année académique 2011-2012 Dissertation présentée par Aurélie Albert en vue de l’obtention du grade de Docteur en Sciences Faculté des Sciences Département de Biologie, Ecologie et Environnement Unité de Biologie du Comportement, Ethologie et Psychologie Animale Feeding and ranging behavior of northern pigtailed macaques ( Macaca leonina ): impact on their seed dispersal effectiveness and ecological contribution in a tropical rainforest at Khao Yai National Park, Thailand ~ ~ ~ Régime alimentaire et déplacements des macaques à queue de cochon ( Macaca leonina ) : impact sur leur efficacité dans la dispersion des graines et sur leur contribution écologique dans une forêt tropicale du parc national de Khao Yai, Thaïlande Année académique 2011-2012 Dissertation présentée par Aurélie Albert en vue de l’obtention du grade de Docteur en Sciences Promotrice : Marie-Claude Huynen (ULg, Belgique) Comité de thèse : Tommaso Savini (KMUTT, Thaïlande) Alain Hambuckers (ULg, Belgique) Pascal Poncin (ULg, Belgique) Président du jury : Jean-Marie Bouquegneau (ULg, Belgique) Membres du jury : Pierre-Michel Forget (MNHN, France) Régine Vercauteren Drubbel (ULB, Belgique) Roseline C. Beudels-Jamar (IRSN, Belgique) Copyright © 2012, Aurélie Albert Toute reproduction du présent document, par quelque procédé que ce soit, ne peut être réalisée qu’avec l’autorisation de l’auteur et du/des promoteur(s). -

Changing Land Use Pattern in Nilgiris Hill Environment Using Geospatial Technology P
Available Online at http://www.recentscientific.com International Journal of Recent Scientific International Journal of Recent Scientific Research Research Vol. 6, Issue, 4, pp.3679-3683, April, 2015 ISSN: 0976-3031 RESEARCH ARTICLE CHANGING LAND USE PATTERN IN NILGIRIS HILL ENVIRONMENT USING GEOSPATIAL TECHNOLOGY P. Thirumalai*, P.H. Anand and J.Murugesan ARTICLE INFODepartment of Geography,ABSTRACT Govt. Arts College (A), Kumbakonam, TamilNadu, India Article History: The Nilgiri mountain range in south India is considered unique by anthropologists, geologists, Received 2nd, March, 2015 climatologists, botanists as well as tourists. It has remained a subject of constant study and research over Received in revised form 10th, the last two centuries. Man-nature balance had continued undisturbed in the Nilgiris for thousands of March, 2015 years until the early 19th century when it became a British colony attracting, in due course, various Accepted 4th, April, 2015 developmental activities. Subsequently, the Nilgiris and its popular hill stations emerged as favourite Published online 28th, places for rest and recuperation, game and for raising commercial plantations. In the process, the April, 2015 traditional indigenous crops were replaced by “English” vegetables and then natural forests gave way to commercial plantations of coffee, tea and other exoticspecies of trees. Land use is the term that is used to describe human uses of the land, or immediate actions modifying or converting land cover. It includes Key words: such broad categories as human settlements, protected areas and agriculture. Within those broad Land use, Hill environment, categories are more refined categories, such as urban and rural settlements, irrigated and rainfed fields, geospatial, English vegetables, national parks and forest reserves, and transportation and other infrastructure. -

Mitochondrial and Nuclear Markers Suggest Hanuman Langur (Primates: Colobinae) Polyphyly: Implications for Their Species Status
Molecular Phylogenetics and Evolution 54 (2010) 627–633 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Short Communication Mitochondrial and nuclear markers suggest Hanuman langur (Primates: Colobinae) polyphyly: Implications for their species status K. Praveen Karanth a,c,*, Lalji Singh b, Caro-Beth Stewart c a Centre for Ecological Sciences, Indian Institute of Science, Bangalore 560012, India b Center for Cellular and Molecular Biology, Uppal Road, Hyderabad 500007, India c Department of Biological Sciences, University at Albany, State University of New York, Albany, NY 12222, USA article info abstract Article history: Recent molecular studies on langurs of the Indian subcontinent suggest that the widely-distributed and Received 30 June 2008 morphologically variable Hanuman langurs (Semnopithecus entellus) are polyphyletic with respect to Revised 9 October 2009 Nilgiri and purple-faced langurs. To further investigate this scenario, we have analyzed additional Accepted 29 October 2009 sequences of mitochondrial cytochrome b as well as nuclear protamine P1 genes from these species. Available online 6 November 2009 The results confirm Hanuman langur polyphyly in the mitochondrial tree and the nuclear markers sug- gest that the Hanuman langurs share protamine P1 alleles with Nilgiri and purple-faced langurs. We rec- Keywords: ommend provisional splitting of the so-called Hanuman langurs into three species such that the Semnopithecus taxonomy is consistent with -

Nilgiri Langur: Biology and Status 1 2
National Studbook of Nilgiri Langur (Trachypithecus johnii) May, 2011 National Studbook of Nilgiri Langur (Trachypithecus johnii) Studbook compiled and analysed by Manjari Malviya Anupam Srivastav Parag Nigam P. C. Tyagi May, 2011 Copyright © WII, Dehradun and CZA, New Delhi, 2011 Cover Photo: Dr. H.N. Kumara This report may be quoted freely but the source must be acknowledged and cited as: Malviya. M., Srivastav, A., Nigam. P, and Tyagi. P.C., 2011. Indian National Studbook of Nilgiri Langur (Trachypithecus johnii). Wildlife Institute of India, Dehradun and Central Zoo Authority, New Delhi. Published as Technical Report of the CZA assignment for compilation and publication of Indian National Studbooks for selected endangered species of wild animals in Indian zoos. Acknowledgements This studbook is a part of the Central Zoo Authority, New Delhi, assignment to the Wildlife Institute of India, Dehradun for the compilation and publication of studbooks of selected endangered faunal types in Indian zoos. The authors wish to thank the Central Zoo Authority for financial support and the opportunity to compile the National Studbook for Nilgiri Langur. We are thankful to Shri. P. R. Sinha, Director, WII for his guidance and support. We would also like to express our appreciation for the advice and support extended by Dr. V. B. Mathur, Dean Faculty of Wildlife Sciences, WII. The authors also wish to thank Shri. B.S. Bonal, Member Secretary, CZA, Dr. B.K. Gupta, Evaluation and monitoring officer, Dr. Naeem Akhtar, Scientific Officer and Mr. Vivek Goel, Data Processing Assistant, CZA for their kind support. The help of the following zoos holding Nilgiri langur in captivity in India is gratefully acknowledged in compilation of data for the studbook. -

F a C T S H E
F A C T S H E E T Recent Primate Incidents Demonstrate Risks To Public Health and Safety, Animal Welfare November 2009 (Indiana): A woman was holding her 10-month-old granddaughter near an enclosure where a monkey was kept as a pet. The monkey pulled the hood of the girl’s coat, causing her head to strike the metal cage, and pulled her hair. The child was taken to the hospital and released that night. November 2009 (Tennessee): A capuchin monkey was found on a road. He had escaped from an SUV of a family vacationing in the area while they were eating at a restaurant, and was recaptured. November 2009 (Florida): A macaque monkey was on the loose outside a Pinellas County apartment complex. October 2009 (Kentucky): Authorities found a baboon being kept in the garage of a Kenton County home. The owners said they bought the animal from an Ohio dealer, and they surrendered her to a sanctuary. September 2009 (Florida): Authorities were looking for a pet patas monkey who had escaped from a Marion County home and been on the loose about two months. June 2009 (New Hampshire): An employee of a farm was severely bitten by a macaque monkey after leaving an enclosure unsecured. Macaques often carry Herpes B virus, and research published by the CDC concludes the health risk makes macaques unsuitable as pets. April 2009 (Oregon): A man brought a capuchin monkey in a diaper to a park. A 6-year-old girl approached and the animal jumped on her, causing two puncture wounds below her eye. -

Ranging Pattern in Bonnet Macaque (Macaca Radiata)
Ranging Pattern in Bonnet Macaque (Macaca radiata) A thesis submitted to Indian Institute of Science Education and Research, Pune in partial fulfillment of the requirements for the BS-MS Dual Degree Programme by Karan Chandrakant Gajbe Indian Institute of Science Education and Research Pune Dr. Homi Bhabha Road Pashan, Pune 411008, India April 2018 Supervisor: Dr. H. N. Kumara ©Karan Gajbe 2018 All rights reserved 1 \ 2 DECLARATION I hereby declare that the matter embodied in the report entitled “Ranging pattern in Bonnet macaque (Macaca radiata)” are the results of the work carried out by me at the Department of Conservation Biology, Sálim Ali Center for Ornithology and Natural history (SACON), under the supervision of Dr. H. N. Kumara and the same has not been submitted elsewhere for any other degree. 3 ABSTRACT: Patterns of ranging behavior of a species depend on distribution and abundance of different resources including food resources and roosting trees, further also depend on group size, intergroup encounters, phenology and movements of the group on previous days, season variability and rainfall. If a species is exposed to humans and live commensal with humans, then the degree of provisioning influences their ecology which includes activity and ranging pattern of that species. To understand this phenomenon, I selected Bonnet Macaque (Macaca radiata) as a model organism to understand the ranging pattern where there are developmental activities and alteration to their habitat. For the study, four groups of bonnet macaque were selected from Chamundi Hills, Mysore, Karnataka. I have collected 12:00 hrs of observations for 106 days over 7 non- consecutive months (June 2017- February 2018) of study.