Cjz 2016 0142 Supplement 3.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

P0455-P0459.Pdf
The Condor 101:455-459 0 The Cooper Ornithological Society 1999 BOOK REVIEWS Social Influences on Vocal Development.- Why read the rest of the book if the main themes Charles T Snowdon and Mat-tine Hausberger, eds. are already discussed in the introduction? I believe it 1997. Cambridge University Press, Cambridge, U.K. is worth it, not only to check the statements of the ix + 351 pp. ISBN 0521 49526 1. $95.00 (cloth). editors. All the articles are fine reviews of a given At least in certain aspects, this book is a referee’s field, written by experts. Doug Nelson provides a very dream: not only do the editors provide short summaries good account of the state of the art in song learning in their introduction of the 16 articles making up this theory, and as I expressed above, I fully agree with his volume, they also inform the reader about the main claim that the two stage nature of song learning is still general conclusions that can be drawn from those ar- not fully understood or accepted by other researchers ticles. According to Snowdon and Hausberger, there of the field. are five central themes that arc treated in almost all Luis Baptista and Sandra Gaunt provide a very com- contributions. The first and main theme is the claim petent review on social interaction and vocal devel- that vocal learning has more aspects than learning to opment in birds. As ever, there is a wealth of findings produce sounds. It is also necessary to learn how to from the lab and from the field included in their re- use and comprehend vocalizations. -
1 ID Euring Latin Binomial English Name Phenology Galliformes
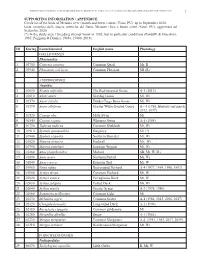
BIRDS OF METAURO RIVER: A GREAT ORNITHOLOGICAL DIVERSITY IN A SMALL ITALIAN URBANIZING BIOTOPE, REQUIRING GREATER PROTECTION 1 SUPPORTING INFORMATION / APPENDICE Check list of the birds of Metauro river (mouth and lower course / Fano, PU), up to September 2020. Lista completa delle specie ornitiche del fiume Metauro (foce e basso corso /Fano, PU), aggiornata ad Settembre 2020. (*) In the study area 1 breeding attempt know in 1985, but in particolar conditions (Pandolfi & Giacchini, 1985; Poggiani & Dionisi, 1988a, 1988b, 2019). ID Euring Latin binomial English name Phenology GALLIFORMES Phasianidae 1 03700 Coturnix coturnix Common Quail Mr, B 2 03940 Phasianus colchicus Common Pheasant SB (R) ANSERIFORMES Anatidae 3 01690 Branta ruficollis The Red-breasted Goose A-1 (2012) 4 01610 Anser anser Greylag Goose Mi, Wi 5 01570 Anser fabalis Tundra/Taiga Bean Goose Mi, Wi 6 01590 Anser albifrons Greater White-fronted Goose A – 4 (1986, february and march 2012, 2017) 7 01520 Cygnus olor Mute Swan Mi 8 01540 Cygnus cygnus Whooper Swan A-1 (1984) 9 01730 Tadorna tadorna Common Shelduck Mr, Wi 10 01910 Spatula querquedula Garganey Mr (*) 11 01940 Spatula clypeata Northern Shoveler Mr, Wi 12 01820 Mareca strepera Gadwall Mr, Wi 13 01790 Mareca penelope Eurasian Wigeon Mr, Wi 14 01860 Anas platyrhynchos Mallard SB, Mr, W (R) 15 01890 Anas acuta Northern Pintail Mi, Wi 16 01840 Anas crecca Eurasian Teal Mr, W 17 01960 Netta rufina Red-crested Pochard A-4 (1977, 1994, 1996, 1997) 18 01980 Aythya ferina Common Pochard Mr, W 19 02020 Aythya nyroca Ferruginous -

AOU Classification Committee – North and Middle America
AOU Classification Committee – North and Middle America Proposal Set 2016-C No. Page Title 01 02 Change the English name of Alauda arvensis to Eurasian Skylark 02 06 Recognize Lilian’s Meadowlark Sturnella lilianae as a separate species from S. magna 03 20 Change the English name of Euplectes franciscanus to Northern Red Bishop 04 25 Transfer Sandhill Crane Grus canadensis to Antigone 05 29 Add Rufous-necked Wood-Rail Aramides axillaris to the U.S. list 06 31 Revise our higher-level linear sequence as follows: (a) Move Strigiformes to precede Trogoniformes; (b) Move Accipitriformes to precede Strigiformes; (c) Move Gaviiformes to precede Procellariiformes; (d) Move Eurypygiformes and Phaethontiformes to precede Gaviiformes; (e) Reverse the linear sequence of Podicipediformes and Phoenicopteriformes; (f) Move Pterocliformes and Columbiformes to follow Podicipediformes; (g) Move Cuculiformes, Caprimulgiformes, and Apodiformes to follow Columbiformes; and (h) Move Charadriiformes and Gruiformes to precede Eurypygiformes 07 45 Transfer Neocrex to Mustelirallus 08 48 (a) Split Ardenna from Puffinus, and (b) Revise the linear sequence of species of Ardenna 09 51 Separate Cathartiformes from Accipitriformes 10 58 Recognize Colibri cyanotus as a separate species from C. thalassinus 11 61 Change the English name “Brush-Finch” to “Brushfinch” 12 62 Change the English name of Ramphastos ambiguus 13 63 Split Plain Wren Cantorchilus modestus into three species 14 71 Recognize the genus Cercomacroides (Thamnophilidae) 15 74 Split Oceanodroma cheimomnestes and O. socorroensis from Leach’s Storm- Petrel O. leucorhoa 2016-C-1 N&MA Classification Committee p. 453 Change the English name of Alauda arvensis to Eurasian Skylark There are a dizzying number of larks (Alaudidae) worldwide and a first-time visitor to Africa or Mongolia might confront 10 or more species across several genera. -

An Update of Wallacels Zoogeographic Regions of the World
REPORTS To examine the temporal profile of ChC produc- specification of a distinct, and probably the last, 3. G. A. Ascoli et al., Nat. Rev. Neurosci. 9, 557 (2008). tion and their correlation to laminar deployment, cohort in this lineage—the ChCs. 4. J. Szentágothai, M. A. Arbib, Neurosci. Res. Program Bull. 12, 305 (1974). we injected a single pulse of BrdU into pregnant A recent study demonstrated that progeni- CreER 5. P. Somogyi, Brain Res. 136, 345 (1977). Nkx2.1 ;Ai9 females at successive days be- tors below the ventral wall of the lateral ventricle 6. L. Sussel, O. Marin, S. Kimura, J. L. Rubenstein, tween E15 and P1 to label mitotic progenitors, (i.e., VGZ) of human infants give rise to a medial Development 126, 3359 (1999). each paired with a pulse of tamoxifen at E17 to migratory stream destined to the ventral mPFC 7. S. J. Butt et al., Neuron 59, 722 (2008). + 18 8. H. Taniguchi et al., Neuron 71, 995 (2011). label NKX2.1 cells (Fig. 3A). We first quanti- ( ). Despite species differences in the develop- 9. L. Madisen et al., Nat. Neurosci. 13, 133 (2010). fied the fraction of L2 ChCs (identified by mor- mental timing of corticogenesis, this study and 10. J. Szabadics et al., Science 311, 233 (2006). + phology) in mPFC that were also BrdU+. Although our findings raise the possibility that the NKX2.1 11. A. Woodruff, Q. Xu, S. A. Anderson, R. Yuste, Front. there was ChC production by E15, consistent progenitors in VGZ and their extended neurogenesis Neural Circuits 3, 15 (2009). -

Leptosomiformes ~ Trogoniformes ~ Bucerotiformes ~ Piciformes
Birds of the World part 6 Afroaves The core landbirds originating in Africa TELLURAVES: AFROAVES – core landbirds originating in Africa (8 orders) • ORDER ACCIPITRIFORMES – hawks and allies (4 families, 265 species) – Family Cathartidae – New World vultures (7 species) – Family Sagittariidae – secretarybird (1 species) – Family Pandionidae – ospreys (2 species) – Family Accipitridae – kites, hawks, and eagles (255 species) • ORDER STRIGIFORMES – owls (2 families, 241 species) – Family Tytonidae – barn owls (19 species) – Family Strigidae – owls (222 species) • ORDER COLIIFORMES (1 family, 6 species) – Family Coliidae – mousebirds (6 species) • ORDER LEPTOSOMIFORMES (1 family, 1 species) – Family Leptosomidae – cuckoo-roller (1 species) • ORDER TROGONIFORMES (1 family, 43 species) – Family Trogonidae – trogons (43 species) • ORDER BUCEROTIFORMES – hornbills and hoopoes (4 families, 74 species) – Family Upupidae – hoopoes (4 species) – Family Phoeniculidae – wood hoopoes (9 species) – Family Bucorvidae – ground hornbills (2 species) – Family Bucerotidae – hornbills (59 species) • ORDER PICIFORMES – woodpeckers and allies (9 families, 443 species) – Family Galbulidae – jacamars (18 species) – Family Bucconidae – puffbirds (37 species) – Family Capitonidae – New World barbets (15 species) – Family Semnornithidae – toucan barbets (2 species) – Family Ramphastidae – toucans (46 species) – Family Megalaimidae – Asian barbets (32 species) – Family Lybiidae – African barbets (42 species) – Family Indicatoridae – honeyguides (17 species) – Family -

Asynchronous Evolution of Interdependent Nest Characters Across the Avian Phylogeny
ARTICLE DOI: 10.1038/s41467-018-04265-x OPEN Asynchronous evolution of interdependent nest characters across the avian phylogeny Yi-Ting Fang1, Mao-Ning Tuanmu 2 & Chih-Ming Hung 2 Nest building is a widespread behavior among birds that reflects their adaptation to the environment and evolutionary history. However, it remains unclear how nests evolve and how their evolution relates to the bird phylogeny. Here, by examining the evolution of three nest — — 1234567890():,; characters structure, site, and attachment across all bird families, we reveal that nest characters did not change synchronically across the avian phylogeny but had disparate evolutionary trajectories. Nest structure shows stronger phylogenetic signal than nest site, while nest attachment has little variation. Nevertheless, the three characters evolved inter- dependently. For example, the ability of birds to explore new nest sites might depend on the emergence of novel nest structure and/or attachment. Our results also reveal labile nest characters in passerines compared with other birds. This study provides important insights into avian nest evolution and suggests potential associations between nest diversification and the adaptive radiations that generated modern bird lineages. 1 Department of Life Sciences, National Chung Hsing University, Taichung, Taiwan. 2 Biodiversity Research Center, Academia Sinica, Taipei, Taiwan. Correspondence and requests for materials should be addressed to M.-N.T. (email: [email protected]) or to C.-M.H. (email: [email protected]) NATURE COMMUNICATIONS | (2018) 9:1863 | DOI: 10.1038/s41467-018-04265-x | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/s41467-018-04265-x lmost all birds build nests, ranging from a simple scratch attachment. -

Journal of Avian Biology JAV-00869 Wang, N
Journal of Avian Biology JAV-00869 Wang, N. and Kimball, R. T. 2016. Re-evaluating the distribution of cooperative breeding in birds: is it tightly linked with altriciality? – J. Avian Biol. doi: 10.1111/jav.00869 Supplementary material Appendix 1. Table A1. The characteristics of the 9993 species based on Jetz et al. (2012) Order Species Criteria1 Developmental K K+S K+S+I LB Mode ACCIPITRIFORMES Accipiter albogularis 0 0 0 0 1 ACCIPITRIFORMES Accipiter badius 0 0 0 0 1 ACCIPITRIFORMES Accipiter bicolor 0 0 0 0 1 ACCIPITRIFORMES Accipiter brachyurus 0 0 0 0 1 ACCIPITRIFORMES Accipiter brevipes 0 0 0 0 1 ACCIPITRIFORMES Accipiter butleri 0 0 0 0 1 ACCIPITRIFORMES Accipiter castanilius 0 0 0 0 1 ACCIPITRIFORMES Accipiter chilensis 0 0 0 0 1 ACCIPITRIFORMES Accipiter chionogaster 0 0 0 0 1 ACCIPITRIFORMES Accipiter cirrocephalus 0 0 0 0 1 ACCIPITRIFORMES Accipiter collaris 0 0 0 0 1 ACCIPITRIFORMES Accipiter cooperii 0 0 0 0 1 ACCIPITRIFORMES Accipiter erythrauchen 0 0 0 0 1 ACCIPITRIFORMES Accipiter erythronemius 0 0 0 0 1 ACCIPITRIFORMES Accipiter erythropus 0 0 0 0 1 ACCIPITRIFORMES Accipiter fasciatus 0 0 0 0 1 ACCIPITRIFORMES Accipiter francesiae 0 0 0 0 1 ACCIPITRIFORMES Accipiter gentilis 0 0 0 0 1 ACCIPITRIFORMES Accipiter griseiceps 0 0 0 0 1 ACCIPITRIFORMES Accipiter gularis 0 0 0 0 1 ACCIPITRIFORMES Accipiter gundlachi 0 0 0 0 1 ACCIPITRIFORMES Accipiter haplochrous 0 0 0 0 1 ACCIPITRIFORMES Accipiter henicogrammus 0 0 0 0 1 ACCIPITRIFORMES Accipiter henstii 0 0 0 0 1 ACCIPITRIFORMES Accipiter imitator 0 0 0 0 1 ACCIPITRIFORMES -

Silvery-Cheeked Hornbill Bycanistes Brevis
Silvery-cheeked Hornbill Bycanistes brevis Class: Aves Order: Bucerotiformes Family: Bucerotidae Characteristics: A fairly large bird with a cream-colored, lightweight casque (“horn”) on top of its beak. The head has silvery-grey feathers (hence the name) and the rest of the body is covered with black or white feathers. In males, the casque is larger than the females. Otherwise they are quite similar. Behavior: They usually live in pairs and, as they are fairly gregarious, will sometimes roost in flocks of several hundred individuals (Beauty of Birds). They have a fairly wide range through which they fly long distances feeding on fruits and small birds in flight. They return to their tree roost at night. Reproduction: They breed in the African spring (September and October). The female finds a cavity and, with the male’s help, seals herself in leaving a small hole through which he feeds her regurgitated fruit. She lays a clutch of 1-3 eggs which hatch following a 40-day incubation period (Oiseaux Birds). Diet: Wild: mainly fruits but will also eat insects, small birds, rodents, small reptiles, centipedes Zoo: softbill, fruit/veg mix, hardboiled egg, dog food and parrot pellets Conservation: The population appears to be quite stable and has a large range, however their numbers seem to be declining in Zimbabwe (Biodiversity Explorer). FYI: The hornbill is one of the few birds that have eyelashes to shield them from sun and dust (Pittsburg Zoo). . -

Preliminary Survey on Relative Diversity and Residential Status of Avifauna in District Karnal, Haryana ( India)
International Journal of Advanced Scientific Research and Management, Volume 4 Issue 6, June 2019 www.ijasrm.com ISSN 2455-6378 Preliminary Survey on Relative Diversity and Residential Status of Avifauna in district Karnal, Haryana ( India). Parveen Kumar Vats Associate Professor, Department of Zoology,Pt. C.L.S. Govt. College Karnal Karnal, Haryana, India Abstract transferring nutrients and spores from one place to The present study was conducted in another during their migration and local movements different sites of district Karnal, Haryana (India) (Niemi, 1985). Birds show variations greatly in their from May 2017 to April 2019. A total of 122 bird diversity, habitats, abundance and distribution species were observed by using line and point count throughout the globe. They have usually more method. These 122 bird species belongs to 17 diversity in tropics than others regions like different orders and maximum bird species were temperate, alpine or polar. Their habitat preferences observed in order Passeriformes (48) followed by are more or less specialized. They occupy higher order Pelecaniformes (14). Accipitridae and trophic levels in food webs. They also vary in their Ardeidae were two families in which equal number abundance because some species occur in large of bird species (8) were observed. A total of 88 bird numbers while others are represented by few species out of 122 observed avian species were individuals only. Some avian species have small resident, 29 bird species were winter migrants and 5 breeding ranges in some particular region whereas bird species were observed only in summer during others travel long distance and annual migrations the present study. -

Avian Genomics : Fledging Into the Wild!
Erschienen in: Journal of Ornithology ; 156 (2015), 4. - S. 851-865 https://dx.doi.org/10.1007/s10336-015-1253-y Avian genomics: fledging into the wild! Robert H. S. Kraus1,2 • Michael Wink3 Abstract Next generation sequencing (NGS) technolo- Zusammenfassung gies provide great resources to study bird evolution and avian functional genomics. They also allow for the iden- Die Ornithologie ist im Zeitalter der Genomik ange- tification of suitable high-resolution markers for detailed kommen analyses of the phylogeography of a species or the con- nectivity of migrating birds between breeding and winter- Neue Sequenziertechnologien (Next Generation Sequen- ing populations. This review discusses the application of cing; NGS) ero¨ffnen die Mo¨glichkeit, Evolution und DNA markers for the study of systematics and phylogeny, funktionelle Genomik bei Vo¨geln umfassend zu untersu- but also population genetics and phylogeography. chen. Weiterhin erlaubt die NGS-Technologie, geeignete, Emphasis in this review is on the new methodology of hochauflo¨sende Markersysteme fu¨r Mikrosatelliten und NGS and its use to study avian genomics. The recent Single Nucleotide Polymorphisms (SNPs) zu identifizieren, publication of the first phylogenomic tree of birds based on um detaillierte Analysen zur Phylogeographie einer Art genome data of 48 bird taxa from 34 orders is presented in oder zur Konnektivita¨t von Zugvo¨geln zwischen Brut- und more detail. Winterpopulationen durchzufu¨hren. Dieses Review widmet sich der Anwendung von DNA Markern fu¨r die Erfor- Keywords Next generation sequencing Á Genetics Á schung von Systematik und Phylogenie sowie Populati- Ornithology Á Whole genome Á Phylogenomics Á Single onsgenetik und Phylogeographie. -

The Origin and Diversification of Birds
Current Biology Review The Origin and Diversification of Birds Stephen L. Brusatte1,*, Jingmai K. O’Connor2,*, and Erich D. Jarvis3,4,* 1School of GeoSciences, University of Edinburgh, Grant Institute, King’s Buildings, James Hutton Road, Edinburgh EH9 3FE, UK 2Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China 3Department of Neurobiology, Duke University Medical Center, Durham, NC 27710, USA 4Howard Hughes Medical Institute, Chevy Chase, MD 20815, USA *Correspondence: [email protected] (S.L.B.), [email protected] (J.K.O.), [email protected] (E.D.J.) http://dx.doi.org/10.1016/j.cub.2015.08.003 Birds are one of the most recognizable and diverse groups of modern vertebrates. Over the past two de- cades, a wealth of new fossil discoveries and phylogenetic and macroevolutionary studies has transformed our understanding of how birds originated and became so successful. Birds evolved from theropod dino- saurs during the Jurassic (around 165–150 million years ago) and their classic small, lightweight, feathered, and winged body plan was pieced together gradually over tens of millions of years of evolution rather than in one burst of innovation. Early birds diversified throughout the Jurassic and Cretaceous, becoming capable fliers with supercharged growth rates, but were decimated at the end-Cretaceous extinction alongside their close dinosaurian relatives. After the mass extinction, modern birds (members of the avian crown group) explosively diversified, culminating in more than 10,000 species distributed worldwide today. Introduction dinosaurs Dromaeosaurus albertensis or Troodon formosus.This Birds are one of the most conspicuous groups of animals in the clade includes all living birds and extinct taxa, such as Archaeop- modern world. -

Temperature Drives the Evolution and Global Distribution of Avian Eggshell Colour 2
bioRxiv preprint doi: https://doi.org/10.1101/559435; this version posted February 24, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Temperature drives the evolution and global distribution of avian eggshell colour 2 3 Phillip A. Wisocki1, Patrick Kennelly1, Indira Rojas Rivera1, Phillip Cassey2, Daniel Hanley1* 4 5 1Long Island University – Post, 720 Northern Boulevard, Brookville, NY 11548, USA 6 2School of Earth and Environmental Sciences, University of Adelaide, SA 5005, Australia 7 8 *email: [email protected] 9 The survival of a bird’s egg depends upon their ability to maintain within strict thermal 10 limits. Avian eggshell colours have long been considered a phenotype that can help them stay 11 within these thermal limits, with dark eggs absorbing heat more rapidly than bright eggs. 12 Although long disputed, evidence suggests that darker eggs do increase in temperature more 13 rapidly than lighter eggs, explaining why dark eggs are often considered as a cost to trade- 14 off against crypsis. Although studies have considered whether eggshell colours can confer an 15 adaptive benefit, no study has demonstrated evidence that eggshell colours have actually 16 adapted for this function. This would require data spanning a wide phylogenetic diversity of 17 birds and a global spatial scale. Here we show evidence that darker and browner eggs have 18 indeed evolved in cold climes, and that the thermoregulatory advantage for avian eggs is a 19 stronger selective pressure in cold climates.