Temperature Drives the Evolution and Global Distribution of Avian Eggshell Colour 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AOU Classification Committee – North and Middle America
AOU Classification Committee – North and Middle America Proposal Set 2016-C No. Page Title 01 02 Change the English name of Alauda arvensis to Eurasian Skylark 02 06 Recognize Lilian’s Meadowlark Sturnella lilianae as a separate species from S. magna 03 20 Change the English name of Euplectes franciscanus to Northern Red Bishop 04 25 Transfer Sandhill Crane Grus canadensis to Antigone 05 29 Add Rufous-necked Wood-Rail Aramides axillaris to the U.S. list 06 31 Revise our higher-level linear sequence as follows: (a) Move Strigiformes to precede Trogoniformes; (b) Move Accipitriformes to precede Strigiformes; (c) Move Gaviiformes to precede Procellariiformes; (d) Move Eurypygiformes and Phaethontiformes to precede Gaviiformes; (e) Reverse the linear sequence of Podicipediformes and Phoenicopteriformes; (f) Move Pterocliformes and Columbiformes to follow Podicipediformes; (g) Move Cuculiformes, Caprimulgiformes, and Apodiformes to follow Columbiformes; and (h) Move Charadriiformes and Gruiformes to precede Eurypygiformes 07 45 Transfer Neocrex to Mustelirallus 08 48 (a) Split Ardenna from Puffinus, and (b) Revise the linear sequence of species of Ardenna 09 51 Separate Cathartiformes from Accipitriformes 10 58 Recognize Colibri cyanotus as a separate species from C. thalassinus 11 61 Change the English name “Brush-Finch” to “Brushfinch” 12 62 Change the English name of Ramphastos ambiguus 13 63 Split Plain Wren Cantorchilus modestus into three species 14 71 Recognize the genus Cercomacroides (Thamnophilidae) 15 74 Split Oceanodroma cheimomnestes and O. socorroensis from Leach’s Storm- Petrel O. leucorhoa 2016-C-1 N&MA Classification Committee p. 453 Change the English name of Alauda arvensis to Eurasian Skylark There are a dizzying number of larks (Alaudidae) worldwide and a first-time visitor to Africa or Mongolia might confront 10 or more species across several genera. -

An Update of Wallacels Zoogeographic Regions of the World
REPORTS To examine the temporal profile of ChC produc- specification of a distinct, and probably the last, 3. G. A. Ascoli et al., Nat. Rev. Neurosci. 9, 557 (2008). tion and their correlation to laminar deployment, cohort in this lineage—the ChCs. 4. J. Szentágothai, M. A. Arbib, Neurosci. Res. Program Bull. 12, 305 (1974). we injected a single pulse of BrdU into pregnant A recent study demonstrated that progeni- CreER 5. P. Somogyi, Brain Res. 136, 345 (1977). Nkx2.1 ;Ai9 females at successive days be- tors below the ventral wall of the lateral ventricle 6. L. Sussel, O. Marin, S. Kimura, J. L. Rubenstein, tween E15 and P1 to label mitotic progenitors, (i.e., VGZ) of human infants give rise to a medial Development 126, 3359 (1999). each paired with a pulse of tamoxifen at E17 to migratory stream destined to the ventral mPFC 7. S. J. Butt et al., Neuron 59, 722 (2008). + 18 8. H. Taniguchi et al., Neuron 71, 995 (2011). label NKX2.1 cells (Fig. 3A). We first quanti- ( ). Despite species differences in the develop- 9. L. Madisen et al., Nat. Neurosci. 13, 133 (2010). fied the fraction of L2 ChCs (identified by mor- mental timing of corticogenesis, this study and 10. J. Szabadics et al., Science 311, 233 (2006). + phology) in mPFC that were also BrdU+. Although our findings raise the possibility that the NKX2.1 11. A. Woodruff, Q. Xu, S. A. Anderson, R. Yuste, Front. there was ChC production by E15, consistent progenitors in VGZ and their extended neurogenesis Neural Circuits 3, 15 (2009). -

Asynchronous Evolution of Interdependent Nest Characters Across the Avian Phylogeny
ARTICLE DOI: 10.1038/s41467-018-04265-x OPEN Asynchronous evolution of interdependent nest characters across the avian phylogeny Yi-Ting Fang1, Mao-Ning Tuanmu 2 & Chih-Ming Hung 2 Nest building is a widespread behavior among birds that reflects their adaptation to the environment and evolutionary history. However, it remains unclear how nests evolve and how their evolution relates to the bird phylogeny. Here, by examining the evolution of three nest — — 1234567890():,; characters structure, site, and attachment across all bird families, we reveal that nest characters did not change synchronically across the avian phylogeny but had disparate evolutionary trajectories. Nest structure shows stronger phylogenetic signal than nest site, while nest attachment has little variation. Nevertheless, the three characters evolved inter- dependently. For example, the ability of birds to explore new nest sites might depend on the emergence of novel nest structure and/or attachment. Our results also reveal labile nest characters in passerines compared with other birds. This study provides important insights into avian nest evolution and suggests potential associations between nest diversification and the adaptive radiations that generated modern bird lineages. 1 Department of Life Sciences, National Chung Hsing University, Taichung, Taiwan. 2 Biodiversity Research Center, Academia Sinica, Taipei, Taiwan. Correspondence and requests for materials should be addressed to M.-N.T. (email: [email protected]) or to C.-M.H. (email: [email protected]) NATURE COMMUNICATIONS | (2018) 9:1863 | DOI: 10.1038/s41467-018-04265-x | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/s41467-018-04265-x lmost all birds build nests, ranging from a simple scratch attachment. -

Avian Genomics : Fledging Into the Wild!
Erschienen in: Journal of Ornithology ; 156 (2015), 4. - S. 851-865 https://dx.doi.org/10.1007/s10336-015-1253-y Avian genomics: fledging into the wild! Robert H. S. Kraus1,2 • Michael Wink3 Abstract Next generation sequencing (NGS) technolo- Zusammenfassung gies provide great resources to study bird evolution and avian functional genomics. They also allow for the iden- Die Ornithologie ist im Zeitalter der Genomik ange- tification of suitable high-resolution markers for detailed kommen analyses of the phylogeography of a species or the con- nectivity of migrating birds between breeding and winter- Neue Sequenziertechnologien (Next Generation Sequen- ing populations. This review discusses the application of cing; NGS) ero¨ffnen die Mo¨glichkeit, Evolution und DNA markers for the study of systematics and phylogeny, funktionelle Genomik bei Vo¨geln umfassend zu untersu- but also population genetics and phylogeography. chen. Weiterhin erlaubt die NGS-Technologie, geeignete, Emphasis in this review is on the new methodology of hochauflo¨sende Markersysteme fu¨r Mikrosatelliten und NGS and its use to study avian genomics. The recent Single Nucleotide Polymorphisms (SNPs) zu identifizieren, publication of the first phylogenomic tree of birds based on um detaillierte Analysen zur Phylogeographie einer Art genome data of 48 bird taxa from 34 orders is presented in oder zur Konnektivita¨t von Zugvo¨geln zwischen Brut- und more detail. Winterpopulationen durchzufu¨hren. Dieses Review widmet sich der Anwendung von DNA Markern fu¨r die Erfor- Keywords Next generation sequencing Á Genetics Á schung von Systematik und Phylogenie sowie Populati- Ornithology Á Whole genome Á Phylogenomics Á Single onsgenetik und Phylogeographie. -

The Origin and Diversification of Birds
Current Biology Review The Origin and Diversification of Birds Stephen L. Brusatte1,*, Jingmai K. O’Connor2,*, and Erich D. Jarvis3,4,* 1School of GeoSciences, University of Edinburgh, Grant Institute, King’s Buildings, James Hutton Road, Edinburgh EH9 3FE, UK 2Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China 3Department of Neurobiology, Duke University Medical Center, Durham, NC 27710, USA 4Howard Hughes Medical Institute, Chevy Chase, MD 20815, USA *Correspondence: [email protected] (S.L.B.), [email protected] (J.K.O.), [email protected] (E.D.J.) http://dx.doi.org/10.1016/j.cub.2015.08.003 Birds are one of the most recognizable and diverse groups of modern vertebrates. Over the past two de- cades, a wealth of new fossil discoveries and phylogenetic and macroevolutionary studies has transformed our understanding of how birds originated and became so successful. Birds evolved from theropod dino- saurs during the Jurassic (around 165–150 million years ago) and their classic small, lightweight, feathered, and winged body plan was pieced together gradually over tens of millions of years of evolution rather than in one burst of innovation. Early birds diversified throughout the Jurassic and Cretaceous, becoming capable fliers with supercharged growth rates, but were decimated at the end-Cretaceous extinction alongside their close dinosaurian relatives. After the mass extinction, modern birds (members of the avian crown group) explosively diversified, culminating in more than 10,000 species distributed worldwide today. Introduction dinosaurs Dromaeosaurus albertensis or Troodon formosus.This Birds are one of the most conspicuous groups of animals in the clade includes all living birds and extinct taxa, such as Archaeop- modern world. -

BIRDS of BOLIVIA UPDATED SPECIES LIST (Version 03 June 2020) Compiled By: Sebastian K
BIRDS OF BOLIVIA UPDATED SPECIES LIST (Version 03 June 2020) https://birdsofbolivia.org/ Compiled by: Sebastian K. Herzog, Scientific Director, Asociación Armonía ([email protected]) Status codes: R = residents known/expected to breed in Bolivia (includes partial migrants); (e) = endemic; NB = migrants not known or expected to breed in Bolivia; V = vagrants; H = hypothetical (observations not supported by tangible evidence); EX = extinct/extirpated; IN = introduced SACC = South American Classification Committee (http://www.museum.lsu.edu/~Remsen/SACCBaseline.htm) Background shading = Scientific and English names that have changed since Birds of Bolivia (2016, 2019) publication and thus differ from names used in the field guide BoB Synonyms, alternative common names, taxonomic ORDER / FAMILY # Status Scientific name SACC English name SACC plate # comments, and other notes RHEIFORMES RHEIDAE 1 R 5 Rhea americana Greater Rhea 2 R 5 Rhea pennata Lesser Rhea Rhea tarapacensis , Puna Rhea (BirdLife International) TINAMIFORMES TINAMIDAE 3 R 1 Nothocercus nigrocapillus Hooded Tinamou 4 R 1 Tinamus tao Gray Tinamou 5 H, R 1 Tinamus osgoodi Black Tinamou 6 R 1 Tinamus major Great Tinamou 7 R 1 Tinamus guttatus White-throated Tinamou 8 R 1 Crypturellus cinereus Cinereous Tinamou 9 R 2 Crypturellus soui Little Tinamou 10 R 2 Crypturellus obsoletus Brown Tinamou 11 R 1 Crypturellus undulatus Undulated Tinamou 12 R 2 Crypturellus strigulosus Brazilian Tinamou 13 R 1 Crypturellus atrocapillus Black-capped Tinamou 14 R 2 Crypturellus variegatus -

Cjz 2016 0142 Supplement 3.Pdf
Supplementary Figures S1 - S11 PC1 (−) PC1 mean PC1 (+) 30 Figure S1. Shape of eggs associated with 10 20 50 O1 different regions of specific principal components: 5 10 O1 minimal, mean, and maximum. O1 0 0 0 O2 O O2 O O2 O O3 O3 O3 −5 −50 −20 −10 −15 −100 −40 −100 −50 0 50 100 −30 −10 0 10 20 30 −15 −10 −5 0 5 10 15 PC2 (−) PC2 mean PC2 (+) 40 20 20 20 O1 O1 10 O1 0 0 O2 O3O O2 O 0 O2 O O3 −20 O3 −10 −20 −40 −20 −40 −60 −30 −40 −20 0 20 40 −40 −20 0 20 40 −20 −10 0 10 20 PC3 (−) PC3 mean PC3 (+) 30 20 20 O1 20 O1 10 10 O1 0 0 0 O2 O O2 O O2 O O3 O3 O3 −10 −20 −20 −20 −40 −30 −40 −40 −20 0 20 40 −20 0 20 −30 −20 −10 0 10 20 30 15 Figure S2. Position of orders in the space of PC1 and PC2. Shape of eggs correspond to specific parts of PCs. 10 Loadings are shown at the lower left corner of figure. All orders 5 ACCIPITRIFORMES ANSERIFORMES APODIFORMES BUCEROTIFORMES CAPRIMULGIFORMES 0 CARIAMIFORMES CHARADRIIFORMES CICONIIFORMES COLUMBIFORMES PC2 CORACIIFORMES −5 CUCULIFORMES EURYPYGIFORMES FALCONIFORMES GALLIFORMES GAVIIFORMES GRUIFORMES −10 OTIDIFORMES PASSERIFORMES PELECANIFORMES PHOENICOPTERIFORMES PICIFORMES −15 PODICIPEDIFORMES PROCELLARIIFORMES PSITTACIFORMES PTEROCLIDIFORMES SPHENISCIFORMES −20 STRIGIFORMES SULIFORMES −40 −30 −20 −10 0 10 20 PC1 Icz LD PC2 Ilz Iiz PC1 15 30 Figure S3. Position of orders with altricial developmental mode in the space −30 of PC1 and PC2. -
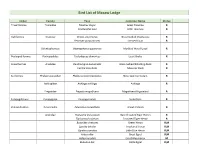
Bird List of Macaw Lodge
Bird List of Macaw Lodge Order Family Taxa Common Name Status Tinamiformes Tnamidae Tinamus major Great Tinamou R Crypturellus soul Little Tinamou R Galliformes Cracidae Ortalis cinereiceps Gray-headed Chachalaca R Penelope purpurascens Crested Guan R Odontophoridae Odontophorus gujanensis Marbled Wood-Quail R Podicipediformes Podicipedidae Tachybaptus dominicus Least Grebe R Anseriformes Anatidae Dendrocygna autumnalis Black-bellied Whistling-Duck R Cairina moschata Muscovy Duck R Suliformes Phalacrocoracidae Phalacrocorax brasilianus Neotropic Cormorant R Anhingidae Anhinga anhinga Anhinga R Fregatidae Fregata magnificens Magnificent Frigatebird R Eurypygiformes Eurypygidae Eurypyga helias Sunbittern R Pelecaniformes Pelecanidae Pelecanus occidentalis Brown Pelican R Ardeidae Tigrisoma mexicanum Bare-throated Tiger-Heron R Tigrisoma fasciatum Fasciated Tiger-Heron R Butorides virescens Green Heron R,M Egretta tricolor Tricolored Heron R,M Egretta caerulea Little Blue Heron R,M Ardea alba Great Egret R,M Ardea herodias Great Blue Heron M Bubulcus ibis Cattle Egret R,M Egretta thula Snowy Egret R,M Nyctanassa violacea Yellow-crowned Night-Heron R,M Cochlearius cochlearius Boat-billed Heron R Threskiornithidae Eudocimus albus White Ibis R Mesembrinibis cayennensis Green Ibis R Platalea ajaja Roseate Sponbill R Charadriiformes Charadriidae Vanellus chilensis Southern Lapwing R Charadrius semipalmatus Semipalmated Plover M Recurvirostridae Himantopus mexicanus Black-necked Stilt R,M Scolopacidae Tringa semipalmata Willet M Tringa solitaria -

Estimating Evolutionary Rates Using Discrete Morphological Characters: a Case
Estimating evolutionary rates using discrete morphological characters: a case study with birds Luke Barrett Harrison Department of Biology McGill University, Montreal April 2013 A thesis submitted to McGill University in partial fulfillment of the requirements of the degree of Doctor of Philosophy © Luke Harrison 2013 DEDICATION I dedicate this thesis to my wife, Soo Bin Chun. I would not have been able to do it without you nor would I have wanted to. semper fidelis & love always TABLE OF CONTENTS ABSTRACT / RÉSUMÉ 1 ACKNOWLEDGEMENTS 5 PREFACE 7 GENERAL INTRODUCTION 10 CHAPTER 1: Estimating Evolutionary Rates of Discrete Morphological Characters Introduction 14 Rates of Phenotypic Evolution 15 Traits, Characters, and Discrete Morphological Characters 16 Heterogeneity in Rates Between Discrete Morphological Characters in Phylogenetic Analysis 18 Absolute Rates of Evolution of Discrete Morphological Characters 20 Westoll (1949) 22 Forey (1988) 22 Cloutier (1991) 23 Wagner (1997) 23 Bromham et al. (2002) 24 Ruta et al. (2006) 24 Brusatte et al. (2008) 25 Roelants et al. (2011) 25 Lloyd et al. (2012) 27 Summary of Previous Methods and Future Directions 28 Appropriate Null Models 28 Likelihood-based Methods for Estimating Morphological Evolutionary Rates: Potential Advantages 29 An Ideal Model-based Framework for Estimating Absolute Rates of Evolution of Discrete Morphological Characters 30 Conclusions 31 CONNECTING TEXT 32 CHAPTER 2: Among-Character Rate Variation in Phylogenetic Analysis of Discrete Morphological Characters: Prevalence and -

Gruiformes ~ Charadriiformes ~ Phaethontiformes ~ Eurypygiformes
Birds of the World part 4 Gruiformes through Eurypygiformes • ORDER GRUIFORMES – cranes, rails, and allies (6 families, 190 species) – Family Sarothruridae – flufftails (12 species) – Family Heliornithidae – finfoots (3 species) – Family Rallidae – rails, crakes, and coots (156 species) – Family Psophiidae – trumpeters (3 species) – Family Gruidae – cranes (15 species) – Family Aramidae – limpkin (1 species) • ORDER CHARADRIIFORMES – shorebirds (19 families, 384 species) – Family Turnicidae – buttonquail (17 species) – Family Burhinidae – stone-curlews or thick-knees (10 species) – Family Chionidae – sheathbills (2 species) – Family Pluvianellidae – Magellanic plover (1 species) – Family Haematopodidae – oystercatchers (12 species) – Family Dromadidae – crab-plover (1 species) – Family Ibidorhynchidae – ibisbill (1 species) – Family Recurvirostridae – stilts and avocets (10 species) – Family Charadriidae – plovers (67 species) – Family Pluvianidae – Egyptian plover (1 species) – Family Rostratulidae – painted-snipes (3 species) – Family Jacanidae – jacanas (8 species) – Family Pedionomidae – plains-wanderer (1 species) – Family Thinocoridae – seedsnipes (4 species) – Family Scolopacidae – sandpipers and snipes (96 species) – Family Glareolidae – coursers and pratincoles (17 species – Family Laridae – gulls, terns, and skimmers (101 species) – Family Stercorariidae – skuas (7 species) – Family Alcidae – auks (25 species) • ORDER PHAETHONTIFORMES (1 family, 3 species) – Family Phaethontidae – tropicbirds (3 species) • ORDER EURYPYGIFORMES -

2018 Guyana Tour Species List
Eagle-Eye Tours Guyana Bird Checklist Guide: Adam Kent March 2018 No. Common Name Scientific Name Heard/Seen Family Order 1 Great Tinamou Tinamus major H Tinamidae (Tinamous) Tinamiformes 2 Little Tinamou Crypturellus soui H Tinamidae (Tinamous) Tinamiformes 3 Undulated Tinamou Crypturellus undulatus H Tinamidae (Tinamous) Tinamiformes 4 Red-legged Tinamou Crypturellus erythropus H Tinamidae (Tinamous) Tinamiformes 5 Variegated Tinamou Crypturellus variegatus H Tinamidae (Tinamous) Tinamiformes 6 White-faced Whistling-Duck Dendrocygna viduata S Anatidae (Ducks, Geese, and Waterfowl) Anseriformes 7 Black-bellied Whistling-Duck Dendrocygna autumnalis S Anatidae (Ducks, Geese, and Waterfowl) Anseriformes 8 Muscovy Duck Cairina moschata S Anatidae (Ducks, Geese, and Waterfowl) Anseriformes 9 Variable Chachalaca Ortalis motmot S Cracidae (Guans, Chachalacas, and Curassows) Galliformes 10 Marail Guan Penelope marail S Cracidae (Guans, Chachalacas, and Curassows) Galliformes 11 Spix's Guan Penelope jacquacu S Cracidae (Guans, Chachalacas, and Curassows) Galliformes 12 Blue-throated Piping-Guan Pipile cumanensis S Cracidae (Guans, Chachalacas, and Curassows) Galliformes 13 Crestless Curassow Mitu tomentosum S Cracidae (Guans, Chachalacas, and Curassows) Galliformes 14 Black Curassow Crax alector S Cracidae (Guans, Chachalacas, and Curassows) Galliformes 15 Crested Bobwhite Colinus cristatus S Odontophoridae (New World Quail) Galliformes 16 Least Grebe Tachybaptus dominicus S Podicipedidae (Grebes) Podicipediformes 17 Maguari Stork Ciconia maguari -

Ancient DNA of New Zealand's Extinct Avifauna
I certify that this work contains no material which has been accepted for the award of any other degree or diploma in my name, in any university or other tertiary institution and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made in the text. In addition, I certify that no part of this work will, in the future, be used in a submission in my name, for any other degree or diploma in any university or other tertiary institution without the prior approval of the University of Adelaide, and where applicable, any partner institution responsible for the joint-award of this degree. I give consent to this copy of my thesis, when deposited in the University Library, being made available for loan and photocopying, subject to the provisions of the Copyright Act 1968. I also give permission for the digital version of my thesis to be made available on the web, via the University’s digital research repository, the Library Search and also through web search engines, unless permission has been granted by the University to restrict access for a period of time. ………………………….. ………………………….. Alexander Boast Date “… a symphony of ‘the most tunable silver sound imaginable’. Aotearoa’s multitudes of birds performed that symphony each dawn for over 60 million years. It was a glorious riot of sound with its own special meaning, for it was a confirmation of the health of a wondrous and unique ecosystem. To my great regret, I arrived in New Zealand in the late twentieth century only to find most of the orchestra seats empty.