Meta-Transcriptomic Analysis of Virus Diversity in Urban Wild Birds With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evidence for Viral Infection in the Copepods Labidocera Aestiva And
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School January 2012 Evidence for Viral Infection in the Copepods Labidocera aestiva and Acartia tonsa in Tampa Bay, Florida Darren Stephenson Dunlap University of South Florida, [email protected] Follow this and additional works at: http://scholarcommons.usf.edu/etd Part of the American Studies Commons, Other Oceanography and Atmospheric Sciences and Meteorology Commons, and the Virology Commons Scholar Commons Citation Dunlap, Darren Stephenson, "Evidence for Viral Infection in the Copepods Labidocera aestiva and Acartia tonsa in Tampa Bay, Florida" (2012). Graduate Theses and Dissertations. http://scholarcommons.usf.edu/etd/4032 This Thesis is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Evidence of Viruses in the Copepods Labidocera aestiva and Acartia tonsa in Tampa Bay, Florida By Darren S. Dunlap A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science College of Marine Science University of South Florida Major Professor: Mya Breitbart, Ph.D Kendra Daly, Ph.D Ian Hewson, Ph.D Date of Approval: March 19, 2012 Key Words: Copepods, Single-stranded DNA Viruses, Mesozooplankton, Transmission Electron Microscopy, Metagenomics Copyright © 2012, Darren Stephenson Dunlap DEDICATION None of this would have been possible without the generous love and support of my entire family over the years. My parents, Steve and Jill Dunlap, have always encouraged my pursuits with support and love, and their persistence of throwing me into lakes and rivers is largely responsible for my passion for Marine Science. -

Nucleotide Amino Acid Size (Nt) #Orfs Marnavirus Heterosigma Akashiwo Heterosigma Akashiwo RNA Heterosigma Lang Et Al
Supplementary Table 1: Summary of information for all viruses falling within the seven Marnaviridae genera in our analyses. Accession Genome Genus Species Virus name Strain Abbreviation Source Country Reference Nucleotide Amino acid Size (nt) #ORFs Marnavirus Heterosigma akashiwo Heterosigma akashiwo RNA Heterosigma Lang et al. , 2004; HaRNAV AY337486 AAP97137 8587 One Canada RNA virus 1 virus akashiwo Tai et al. , 2003 Marine single- ASG92540 Moniruzzaman et Classification pending Q sR OV 020 KY286100 9290 Two celled USA ASG92541 al ., 2017 eukaryotes Marine single- Moniruzzaman et Classification pending Q sR OV 041 KY286101 ASG92542 9328 One celled USA al ., 2017 eukaryotes APG78557 Classification pending Wenzhou picorna-like virus 13 WZSBei69459 KX884360 9458 One Bivalve China Shi et al ., 2016 APG78557 Classification pending Changjiang picorna-like virus 2 CJLX30436 KX884547 APG79001 7171 One Crayfish China Shi et al ., 2016 Beihai picorna-like virus 57 BHHQ57630 KX883356 APG76773 8518 One Tunicate China Shi et al ., 2016 Classification pending Beihai picorna-like virus 57 BHJP51916 KX883380 APG76812 8518 One Tunicate China Shi et al ., 2016 Marine single- ASG92530 Moniruzzaman et Classification pending N OV 137 KY130494 7746 Two celled USA ASG92531 al ., 2017 eukaryotes Hubei picorna-like virus 7 WHSF7327 KX884284 APG78434 9614 One Pill worm China Shi et al ., 2016 Classification pending Hubei picorna-like virus 7 WHCC111241 KX884268 APG78407 7945 One Insect China Shi et al ., 2016 Sanxia atyid shrimp virus 2 WHCCII13331 KX884278 APG78424 10445 One Insect China Shi et al ., 2016 Classification pending Freshwater atyid Sanxia atyid shrimp virus 2 SXXX37884 KX883708 APG77465 10400 One China Shi et al ., 2016 shrimp Labyrnavirus Aurantiochytrium single Aurantiochytrium single stranded BAE47143 Aurantiochytriu AuRNAV AB193726 9035 Three4 Japan Takao et al. -

Genetic Content and Evolution of Adenoviruses Andrew J
Journal of General Virology (2003), 84, 2895–2908 DOI 10.1099/vir.0.19497-0 Review Genetic content and evolution of adenoviruses Andrew J. Davison,1 Ma´ria Benko´´ 2 and Bala´zs Harrach2 Correspondence 1MRC Virology Unit, Institute of Virology, Church Street, Glasgow G11 5JR, UK Andrew Davison 2Veterinary Medical Research Institute, Hungarian Academy of Sciences, H-1581 Budapest, [email protected] Hungary This review provides an update of the genetic content, phylogeny and evolution of the family Adenoviridae. An appraisal of the condition of adenovirus genomics highlights the need to ensure that public sequence information is interpreted accurately. To this end, all complete genome sequences available have been reannotated. Adenoviruses fall into four recognized genera, plus possibly a fifth, which have apparently evolved with their vertebrate hosts, but have also engaged in a number of interspecies transmission events. Genes inherited by all modern adenoviruses from their common ancestor are located centrally in the genome and are involved in replication and packaging of viral DNA and formation and structure of the virion. Additional niche-specific genes have accumulated in each lineage, mostly near the genome termini. Capture and duplication of genes in the setting of a ‘leader–exon structure’, which results from widespread use of splicing, appear to have been central to adenovirus evolution. The antiquity of the pre-vertebrate lineages that ultimately gave rise to the Adenoviridae is illustrated by morphological similarities between adenoviruses and bacteriophages, and by use of a protein-primed DNA replication strategy by adenoviruses, certain bacteria and bacteriophages, and linear plasmids of fungi and plants. -

Discovery of a Polyomavirus in European Badgers (Meles Meles) and the Evolution of Host Range in the Family Polyomaviridae
Journal of General Virology (2015), 96, 1411–1422 DOI 10.1099/vir.0.000071 Discovery of a polyomavirus in European badgers (Meles meles) and the evolution of host range in the family Polyomaviridae Sarah C. Hill,13 Aisling A. Murphy,23 Matthew Cotten,3 Anne L. Palser,3 Phillip Benson,2 Sandrine Lesellier,4 Eamonn Gormley,5 Ce´line Richomme,6 Sylvia Grierson,7 Deirdre Ni Bhuachalla,5 Mark Chambers,4,8 Paul Kellam,3,9 Marı´a-Laura Boschiroli,10 Bernhard Ehlers,114 Michael A. Jarvis24 and Oliver G. Pybus14 Correspondence 1Department of Zoology, University of Oxford, UK Bernhard Ehlers 2School of Biomedical and Healthcare Sciences, Plymouth University, UK [email protected] 3 Michael A. Jarvis Wellcome Trust Sanger Institute, UK [email protected] 4Bacteriology Department, Animal and Plant Health Agency, UK Oliver G. Pybus 5School of Veterinary Medicine, University College Dublin (UCD), Ireland [email protected] 6ANSES, Nancy Laboratory for Rabies and Wildlife, France 7Department of Virology, Animal and Plant Health Agency, UK 8School of Veterinary Medicine, University of Surrey, UK 9MRC/UCL Centre for Medical Molecular Virology, University College London, UK 10University Paris-Est, ANSES, Laboratory for Animal Health, Bovine Tuberculosis National Reference Laboratory, France 11Robert Koch Institute, Division 12 ‘Measles, Mumps, Rubella and Viruses Affecting Immunocompromised Patients’, Germany Polyomaviruses infect a diverse range of mammalian and avian hosts, and are associated with a variety of symptoms. However, it is unknown whether the viruses are found in all mammalian families and the evolutionary history of the polyomaviruses is still unclear. Here, we report the discovery of a novel polyomavirus in the European badger (Meles meles), which to our knowledge represents the first polyomavirus to be characterized in the family Mustelidae, and within a European carnivoran. -

And Giant Guitarfish (Rhynchobatus Djiddensis)
VIRAL DISCOVERY IN BLUEGILL SUNFISH (LEPOMIS MACROCHIRUS) AND GIANT GUITARFISH (RHYNCHOBATUS DJIDDENSIS) BY HISTOPATHOLOGY EVALUATION, METAGENOMIC ANALYSIS AND NEXT GENERATION SEQUENCING by JENNIFER ANNE DILL (Under the Direction of Alvin Camus) ABSTRACT The rapid growth of aquaculture production and international trade in live fish has led to the emergence of many new diseases. The introduction of novel disease agents can result in significant economic losses, as well as threats to vulnerable wild fish populations. Losses are often exacerbated by a lack of agent identification, delay in the development of diagnostic tools and poor knowledge of host range and susceptibility. Examples in bluegill sunfish (Lepomis macrochirus) and the giant guitarfish (Rhynchobatus djiddensis) will be discussed here. Bluegill are popular freshwater game fish, native to eastern North America, living in shallow lakes, ponds, and slow moving waterways. Bluegill experiencing epizootics of proliferative lip and skin lesions, characterized by epidermal hyperplasia, papillomas, and rarely squamous cell carcinoma, were investigated in two isolated poopulations. Next generation genomic sequencing revealed partial DNA sequences of an endogenous retrovirus and the entire circular genome of a novel hepadnavirus. Giant Guitarfish, a rajiform elasmobranch listed as ‘vulnerable’ on the IUCN Red List, are found in the tropical Western Indian Ocean. Proliferative skin lesions were observed on the ventrum and caudal fin of a juvenile male quarantined at a public aquarium following international shipment. Histologically, lesions consisted of papillomatous epidermal hyperplasia with myriad large, amphophilic, intranuclear inclusions. Deep sequencing and metagenomic analysis produced the complete genomes of two novel DNA viruses, a typical polyomavirus and a second unclassified virus with a 20 kb genome tentatively named Colossomavirus. -

The Viruses of Wild Pigeon Droppings
The Viruses of Wild Pigeon Droppings Tung Gia Phan1,2, Nguyen Phung Vo1,3,A´ kos Boros4,Pe´ter Pankovics4,Ga´bor Reuter4, Olive T. W. Li6, Chunling Wang5, Xutao Deng1, Leo L. M. Poon6, Eric Delwart1,2* 1 Blood Systems Research Institute, San Francisco, California, United States of America, 2 Department of Laboratory Medicine, University of California San Francisco, San Francisco, California, United States of America, 3 Pharmacology Department, School of Pharmacy, Ho Chi Minh City University of Medicine and Pharmacy, Ho Chi Minh, Vietnam, 4 Regional Laboratory of Virology, National Reference Laboratory of Gastroenteric Viruses, A´ NTSZ Regional Institute of State Public Health Service, Pe´cs, Hungary, 5 Stanford Genome Technology Center, Stanford, California, United States of America, 6 Centre of Influenza Research and School of Public Health, University of Hong Kong, Hong Kong SAR Abstract Birds are frequent sources of emerging human infectious diseases. Viral particles were enriched from the feces of 51 wild urban pigeons (Columba livia) from Hong Kong and Hungary, their nucleic acids randomly amplified and then sequenced. We identified sequences from known and novel species from the viral families Circoviridae, Parvoviridae, Picornaviridae, Reoviridae, Adenovirus, Astroviridae, and Caliciviridae (listed in decreasing number of reads), as well as plant and insect viruses likely originating from consumed food. The near full genome of a new species of a proposed parvovirus genus provisionally called Aviparvovirus contained an unusually long middle ORF showing weak similarity to an ORF of unknown function from a fowl adenovirus. Picornaviruses found in both Asia and Europe that are distantly related to the turkey megrivirus and contained a highly divergent 2A1 region were named mesiviruses. -

Opportunistic Intruders: How Viruses Orchestrate ER Functions to Infect Cells
REVIEWS Opportunistic intruders: how viruses orchestrate ER functions to infect cells Madhu Sudhan Ravindran*, Parikshit Bagchi*, Corey Nathaniel Cunningham and Billy Tsai Abstract | Viruses subvert the functions of their host cells to replicate and form new viral progeny. The endoplasmic reticulum (ER) has been identified as a central organelle that governs the intracellular interplay between viruses and hosts. In this Review, we analyse how viruses from vastly different families converge on this unique intracellular organelle during infection, co‑opting some of the endogenous functions of the ER to promote distinct steps of the viral life cycle from entry and replication to assembly and egress. The ER can act as the common denominator during infection for diverse virus families, thereby providing a shared principle that underlies the apparent complexity of relationships between viruses and host cells. As a plethora of information illuminating the molecular and cellular basis of virus–ER interactions has become available, these insights may lead to the development of crucial therapeutic agents. Morphogenesis Viruses have evolved sophisticated strategies to establish The ER is a membranous system consisting of the The process by which a virus infection. Some viruses bind to cellular receptors and outer nuclear envelope that is contiguous with an intri‑ particle changes its shape and initiate entry, whereas others hijack cellular factors that cate network of tubules and sheets1, which are shaped by structure. disassemble the virus particle to facilitate entry. After resident factors in the ER2–4. The morphology of the ER SEC61 translocation delivering the viral genetic material into the host cell and is highly dynamic and experiences constant structural channel the translation of the viral genes, the resulting proteins rearrangements, enabling the ER to carry out a myriad An endoplasmic reticulum either become part of a new virus particle (or particles) of functions5. -

Molecular Characterization of 52K Protein of Bovine Adenovirus Type 3
MOLECULAR CHARACTERIZATION OF 52K PROTEIN OF BOVINE ADENOVIRUS TYPE 3 A Thesis Submitted to the Faculty of Graduate Studies and Research in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in the Department of Veterinary Microbiology University of Saskatchewan Saskatoon By Carolyn Patricia Paterson © Copyright Carolyn P. Paterson, August 2010. All rights reserved. PERMISSION TO USE In presenting this thesis in partial fulfillment of the requirements for a postgraduate degree from the University of Saskatchewan, I agree that the libraries of this university may make it freely available for inspection. I further agree that permission for copying of this thesis in any manner, whole or in part, for scholarly purposes may be granted by the professors who supervised my thesis work or in their absence, the Head of the Department or the Dean of the college in which my thesis work was done. It is understood that any copying or publication or use of this thesis or parts therof for financial gain shall not be allowed without any written permission. It is also understood that due recognition shall be given to me and to the University of Saskatchewan in any scholarly use which may be made of any material in my thesis. Request for permission to copy or to make other use of material in this thesis in whole or part should be addressed to: Head of the Department of Veterinary Microbiology University of Saskatchewan Saskatoon, Saskatchewan, S7N 5B4 i ABSTRACT Bovine adenovirus (BAdV)-3 is a non-enveloped, icosahedral virus with a double-stranded DNA genome, and is being developed as a vector for vaccination of animals and humans (Rasmussen et al., 1999; Zakhartchouk et al., 1999). -
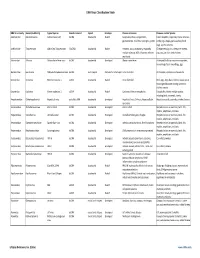
Virus Classification Tables V2.Vd.Xlsx
DNA Virus Classification Table DNA Virus Family Genera (Subfamily) Typical Species Genetic material Capsid Envelope Disease in Humans Diseases in other Species Adenoviridae Mastadenovirus Adenoviruses 1‐47 dsDNA Icosahedral Naked Respiratory illness; conjunctivitis, Canine hepatitis, respiratory illness in horses, gastroenteritis, tonsillitis, meningitis, cystitis cattle, pigs, sheep, goats, sea lions, birds dogs, squirrel enteritis Anelloviridae Torqueviruses Alpha‐Zeta Torqueviruses (‐)ssDNA Icosahedral Naked Hepatitis, lupus, pulmonary, myopathy, Chimpanzee, pig, cow, sheep, tree shrews, multiple sclerosis; 90% of humans infected pigs, cats, sea lions and chickens worldwide Asfarviridae Asfivirus African Swine fever virus dsDNA Icosahedral Enveloped African swine fever Arthropod (tick) transmission or ingestion; hemorrhagic fever in warthogs, pigs Baculoviridae Baculovirus Alpha‐Gamma Baculoviruses dsDNA Stick shaped Occluded or Enveloped none identified Arthropods, Lepidoptera, crustaceans Circoviridae Circovirus Porcine circovirus 1 ssDNA Icosahedral Naked none identified Birds, pigs, dogs; bats; rodents; causes post‐ weaning multisystem wasting syndrome, chicken anemia Circoviridae Cyclovirus Human cyclovirus 1 ssDNA Icosahedral Naked Cyclovirus Vietnam encephalitis Encephalitis; infects multiple species including birds, mammals, insects Hepadnaviridae Orthohepadnavirus Hepatitis B virus partially ssDNA Icosahedral Enveloped Hepatitis B virus; Cirrhosis, Hepatocellular Hepatitis in ducks, squirrels, primates, herons carcinoma Herpesviridae -

Viruses in Transplantation - Not Always Enemies
Viruses in transplantation - not always enemies Virome and transplantation ECCMID 2018 - Madrid Prof. Laurent Kaiser Head Division of Infectious Diseases Laboratory of Virology Geneva Center for Emerging Viral Diseases University Hospital of Geneva ESCMID eLibrary © by author Conflict of interest None ESCMID eLibrary © by author The human virome: definition? Repertoire of viruses found on the surface of/inside any body fluid/tissue • Eukaryotic DNA and RNA viruses • Prokaryotic DNA and RNA viruses (phages) 25 • The “main” viral community (up to 10 bacteriophages in humans) Haynes M. 2011, Metagenomic of the human body • Endogenous viral elements integrated into host chromosomes (8% of the human genome) • NGS is shaping the definition Rascovan N et al. Annu Rev Microbiol 2016;70:125-41 Popgeorgiev N et al. Intervirology 2013;56:395-412 Norman JM et al. Cell 2015;160:447-60 ESCMID eLibraryFoxman EF et al. Nat Rev Microbiol 2011;9:254-64 © by author Viruses routinely known to cause diseases (non exhaustive) Upper resp./oropharyngeal HSV 1 Influenza CNS Mumps virus Rhinovirus JC virus RSV Eye Herpes viruses Parainfluenza HSV Measles Coronavirus Adenovirus LCM virus Cytomegalovirus Flaviviruses Rabies HHV6 Poliovirus Heart Lower respiratory HTLV-1 Coxsackie B virus Rhinoviruses Parainfluenza virus HIV Coronaviruses Respiratory syncytial virus Parainfluenza virus Adenovirus Respiratory syncytial virus Coronaviruses Gastro-intestinal Influenza virus type A and B Human Bocavirus 1 Adenovirus Hepatitis virus type A, B, C, D, E Those that cause -
![Arxiv:2102.00316V2 [Q-Bio.SC] 8 Apr 2021 Dependent RNA-Polymerase (Abbreviated As Rdrp) [2]](https://docslib.b-cdn.net/cover/5691/arxiv-2102-00316v2-q-bio-sc-8-apr-2021-dependent-rna-polymerase-abbreviated-as-rdrp-2-835691.webp)
Arxiv:2102.00316V2 [Q-Bio.SC] 8 Apr 2021 Dependent RNA-Polymerase (Abbreviated As Rdrp) [2]
Polysomally Protected Viruses Michael Wilkinson1;2, David Yllanes1 and Greg Huber1 1 Chan Zuckerberg Biohub, 499 Illinois Street, San Francisco, CA 94158, USA 2 School of Mathematics and Statistics, The Open University,Walton Hall, Milton Keynes, MK7 6AA, UK E-mail: [email protected] January 2021 Abstract. It is conceivable that an RNA virus could use a polysome, that is, a string of ribosomes covering the RNA strand, to protect the genetic material from degradation inside a host cell. This paper discusses how such a virus might operate, and how its presence might be detected by ribosome profiling. There are two possible forms for such a polysomally protected virus, depending upon whether just the forward strand or both the forward and complementary strands can be encased by ribosomes (these will be termed type 1 and type 2, respectively). It is argued that in the type 2 case the viral RNA would evolve an ambigrammatic property, whereby the viral genes are free of stop codons in a reverse reading frame (with forward and reverse codons aligned). Recent observations of ribosome profiles of ambigrammatic narnavirus sequences are consistent with our predictions for the type 2 case. 1. Introduction A canonical model for the structure of a virus [1] consists of genetic material encased in a capsid composed of a protein shell. A simpler model has also been observed, termed a narnavirus (this term is a contraction of `naked RNA virus'). The narnavirus examples that have been characterised appear to be single genes, which code for an RNA- arXiv:2102.00316v2 [q-bio.SC] 8 Apr 2021 dependent RNA-polymerase (abbreviated as RdRp) [2]. -

Diversity and Evolution of Novel Invertebrate DNA Viruses Revealed by Meta-Transcriptomics
viruses Article Diversity and Evolution of Novel Invertebrate DNA Viruses Revealed by Meta-Transcriptomics Ashleigh F. Porter 1, Mang Shi 1, John-Sebastian Eden 1,2 , Yong-Zhen Zhang 3,4 and Edward C. Holmes 1,3,* 1 Marie Bashir Institute for Infectious Diseases and Biosecurity, Charles Perkins Centre, School of Life & Environmental Sciences and Sydney Medical School, The University of Sydney, Sydney, NSW 2006, Australia; [email protected] (A.F.P.); [email protected] (M.S.); [email protected] (J.-S.E.) 2 Centre for Virus Research, Westmead Institute for Medical Research, Westmead, NSW 2145, Australia 3 Shanghai Public Health Clinical Center and School of Public Health, Fudan University, Shanghai 201500, China; [email protected] 4 Department of Zoonosis, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Changping, Beijing 102206, China * Correspondence: [email protected]; Tel.: +61-2-9351-5591 Received: 17 October 2019; Accepted: 23 November 2019; Published: 25 November 2019 Abstract: DNA viruses comprise a wide array of genome structures and infect diverse host species. To date, most studies of DNA viruses have focused on those with the strongest disease associations. Accordingly, there has been a marked lack of sampling of DNA viruses from invertebrates. Bulk RNA sequencing has resulted in the discovery of a myriad of novel RNA viruses, and herein we used this methodology to identify actively transcribing DNA viruses in meta-transcriptomic libraries of diverse invertebrate species. Our analysis revealed high levels of phylogenetic diversity in DNA viruses, including 13 species from the Parvoviridae, Circoviridae, and Genomoviridae families of single-stranded DNA virus families, and six double-stranded DNA virus species from the Nudiviridae, Polyomaviridae, and Herpesviridae, for which few invertebrate viruses have been identified to date.