Effect of Learning to Use a Mobility Aid on Gait and Cognitive Demands in People with Mild to Moderate Alzheimer’S Dementia: Part I - Cane
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
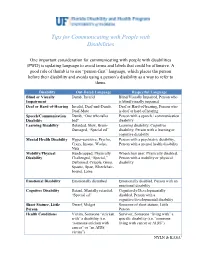
Tips for Communicating with People with Disabilities
Tips for Communicating with People with Disabilities One important consideration for communicating with people with disabilities (PWD) is updating language to avoid terms and labels that could be offensive. A good rule of thumb is to use “person-first” language, which places the person before their disability and avoids using a person’s disability as a way to refer to them. Disability Out-Dated Language Respectful Language Blind or Visually Dumb, Invalid Blind/Visually Impaired, Person who Impairment is blind/visually impaired Deaf or Hard-of-Hearing Invalid, Deaf-and-Dumb, Deaf or Hard-of-hearing, Person who Deaf-Mute is deaf or hard of hearing Speech/Communication Dumb, “One who talks Person with a speech / communication Disability bad" disability Learning Disability Retarded, Slow, Brain- Learning disability, Cognitive Damaged, “Special ed” disability, Person with a learning or cognitive disability Mental Health Disability Hyper-sensitive, Psycho, Person with a psychiatric disability, Crazy, Insane, Wacko, Person with a mental health disability Nuts Mobility/Physical Handicapped, Physically Wheelchair user, Physically disabled, Disability Challenged, “Special,” Person with a mobility or physical Deformed, Cripple, Gimp, disability Spastic, Spaz, Wheelchair- bound, Lame Emotional Disability Emotionally disturbed Emotionally disabled, Person with an emotional disability Cognitive Disability Retard, Mentally retarded, Cognitively/Developmentally “Special ed” disabled, Person with a cognitive/developmental disability Short Stature, Little Dwarf, Midget Someone of short stature, Little Person Person Health Conditions Victim, Someone “stricken Survivor, Someone “living with” a with” a disability (i.e. specific disability (i.e. “someone “someone stricken with living with cancer or AIDS”) cancer” or “an AIDS victim”) NYLN & KASA1 General Tips Use a normal volume and tone when speaking to persons with disabilities. -

Vehicle Acquisition
VEHICLE ACQUISITION F ederal Transit Administration Americans with Disabilities Act Circular C 4710.1 Draft Chapter for Public Comment October 2012 TABLE OF CONTENTS 1 INTRODUCTION 1 1.1 THE DOT ADA REGULATIONS 1 1.2 PROVIDING SERVICE ON BEHALF OF ANOTHER ENTITY: “STAND IN THE SHOES” 1 2 ACQUISITION REQUIREMENTS FOR PUBLIC ENTITIES 3 2.1 BUSES AND VANS 4 2.2 RAPID RAIL AND LIGHT RAIL 5 2.3 COMMUTER RAIL 6 2.4 OTHER CONSIDERATIONS 7 2.5 DEMAND RESPONSIVE SERVICE 8 3 THE MAIN ELEMENTS OF ACCESSIBLE VEHICLES 10 3.1 BUSES AND VANS 13 3.2 RAPID RAIL VEHICLES 17 3.3 LIGHT RAIL VEHICLES 18 3.4 COMMUTER RAIL CARS 21 3.5 OTHER CONSIDERATIONS 23 4 ENSURING THAT VEHICLES ARE COMPLIANT 24 4.1 UNDERSTANDING THE SPECIFICATIONS 24 4.2 OBTAINING PUBLIC INPUT 24 4.3 ADDITIONAL SPECIFICATIONS 25 4.4 INSPECTIONS 25 5 DEFINITIONS 26 6 AUTHORITIES 28 7 REFERENCES 29 APPENDICES APPENDIX 1 SAMPLE BUS AND VAN SPECIFICATION CHECKLIST !"#$#%#$&'()*+,($$ -./')+.$#)0*'1'2'34$&/,52.($6$%(,72$ 8)239.($:;<:$ =,>.$<$37$:? ! 1 INTRODUCTION This Circular chapter on vehicle acquisition serves as a reference document for public transportation providers acquiring vehicles to ensure that these vehicles meet the requirements of the U.S. Department of Transportation (DOT) Americans with Disabilities Act (ADA) regulations. It is the goal of the Federal Transit Administration (FTA) to help transportation providers meet their obligations under the ADA by outlining the regulations, describing effective practices, and presenting the information in an easy-to-use format. Please note that this Circular does not alter, amend, or otherwise affect the DOT ADA regulations themselves; transportation providers are advised to use this Circular in addition to (not in lieu of) the DOT ADA regulations. -

Battery Powered Wheelchair and Mobility Aid Guidance Document
Battery Powered Wheelchair and SafetyMobility requirements applicableAid toGuidance the carriage of battery powered Document wheelchairs and mobility aids when carried by passengers travelling by air Based on the 2019 Regulation compliance with the IATA Dangerous Goods Introduction Regulations. This document is based on the provisions set out in Passengers may only travel with a battery-powered the 2019-2020 Edition of the International Civil mobility aid with the airline’s approval. Proper pre- Aviation Organization (ICAO) Technical Instruction notification by the user helps to ensure that: for the Safe Transport of Dangerous Goods by Air (Technical Instructions) and the 60th Edition of the ▪ all in the transportation chain know what IATA Dangerous Goods Regulations (DGR). device(s) and battery type(s) they are transporting; Information on the DGR can be found here: ▪ how to properly load and handle them; and https://www.iata.org/publications/dgr/Pages/index. ▪ what to do if an incident or accident occurs aspx either in-flight or on the ground. The batteries that power wheelchairs and mobility The pilot-in-command must be informed of the aids are considered dangerous goods when carried location of the mobility aid with installed batteries, by air. These and some other dangerous goods that removed batteries and spare batteries, to best deal are permitted for carriage by passengers can be with any emergencies that may occur. transported safely by air provided certain safety requirements are met. The requirements are Inadvertent operation of battery powered mobility detailed in the IATA Dangerous Goods Regulations, aids can cause friction or electrical load which which are based on the ICAO Technical could lead to a fire. -

2017 Ada Update on Service Animal Rules
2017 ADA UPDATE ON SERVICE ANIMAL RULES The California Hotel & Lodging Association receives many questions regarding the rights and obligations of lodging establishments with respect to service animals. This document contains CHLA’s updated discussion of this topic. First, there has been a significant change regarding California law related to service animals. This change, which is discussed below, will make it easier for lodging operators to deal with guests who claim that their pets are service animals. Here are the basics of California law as it relates to service animals: • There was a time when the California Department of Fair Employment and Housing, which administers California’s Fair Employment and Housing Act (Act), took the position that the Act applied to the lodging industry. At that time, there was concern that lodging operators would have to comply with the more stringent requirements of the Act: (1) it is not limited to dogs— depending on the circumstances, the FEHA protects other types of animals and treats them as service animals; and (2) it provides protections for people who use “comfort” and “companion” animals. Contrary to the Department’s earlier position, however, CHLA was recently advised that the Act only applies to homes, condos, and apartments, and that it does not apply to hotels! • It is important for lodging operators to bear in mind that although the 2011 revisions to the ADA rules regarding service animals expressly state that (1) only dogs qualify as service animals,1 and that (2) “comfort” and “companion” dogs are not service animals,2 if a local jurisdiction in California has broader, more stringent rules than the ADA, the local rules must be complied with. -

Relationship Between Body Image and Physical Functioning Following Rehabilitation for Lower-Limb Amputation
Western University Scholarship@Western Physical Therapy Publications Physical Therapy School 2019 Relationship between body image and physical functioning following rehabilitation for lower-limb amputation Jessica Desrochers Courtney Frengopoulos Michael W.C. Payne Ricardo Viana Susan W. Hunter Follow this and additional works at: https://ir.lib.uwo.ca/ptpub Part of the Physical Therapy Commons Relationship between body image and physical functioning following rehabilitation for lower limb amputation Jessica Desrochers BHSc1; Courtney Frengopoulos MSc2; Michael WC Payne MD3; Ricardo Viana MD3; Susan W Hunter PhD3,4 1. School of Health Studies, University of Western Ontario, London, ON; 2. Faculty of Health & Rehabilitation Sciences, University of Western Ontario, London, ON; 3. Department of Physical Medicine & Rehabilitation, Schulich School of Medicine and Dentistry, University of Western Ontario, London, ON; 4. School of Physical Therapy, University of Western Ontario, London, ON. Running title: Body image and mobility in lower extremity amputees Corresponding author: Dr. Susan W Hunter University of Western Ontario School of Physical Therapy Room 1588, Elborn College 1201 Western Road, London, Ontario Email: [email protected] Telephone: 519-661-2111 ext88845 Funding: This work was supported by the St. Joseph’s Foundation Cognitive Vitality and Brain Health Seed Funding Opportunity. The funding body had no involvement in the conduct of the study. Conflict of interest: None to report. Relationship between body image and physical functioning following rehabilitation for lower limb amputation ABSTRACT Objectives: To evaluate change in body image and the association between body image at discharge and mobility 4 months post rehabilitation. Methods: Prospective cohort of consecutive admissions to inpatient prosthetic rehabilitation. -

The Economics of Enhancing Accessibility Estimating the Benefits and Costs of Participation
The Economics of Enhancing Accessibility Estimating the Benefits and Costs of Participation Discussion01 Paper 2017 • 01 Bridget R.D. Burdett TDG Ltd, Hamilton, New Zealand Stuart M. Locke University of Waikato Management School, Hamilton, New Zealand Frank Scrimgeour Institute for Business Research, University of Waikato, Hamilton, New Zealand The Economics of Enhancing Accessibility Estimating the Benefits and Costs of Participation Discussion Paper No. 2017-01 Prepared for the Roundtable on Economics of Accessible Transport 3-4 March 2016, Paris Bridget R.D. Burdett TDG Ltd, Hamilton, New Zealand Stuart M. Locke Department of Finance, University of Waikato Management School, Hamilton, New Zealand Frank Scrimgeour Institute for Business Research, University of Waikato, Hamilton, New Zealand February 2017 The International Transport Forum The International Transport Forum is an intergovernmental organisation with 57 member countries. It acts as a think tank for transport policy and organises the Annual Summit of transport ministers. ITF is the only global body that covers all transport modes. The ITF is politically autonomous and administratively integrated with the OECD. The ITF works for transport policies that improve peoples’ lives. Our mission is to foster a deeper understanding of the role of transport in economic growth, environmental sustainability and social inclusion and to raise the public profile of transport policy. The ITF organises global dialogue for better transport. We act as a platform for discussion and pre- negotiation of policy issues across all transport modes. We analyse trends, share knowledge and promote exchange among transport decision-makers and civil society. The ITF’s Annual Summit is the world’s largest gathering of transport ministers and the leading global platform for dialogue on transport policy. -

Trams, Similar Vehicles and Systems
TRAMS, SIMILAR VEHICLES AND SYSTEMS Technical Assistance Manual October, 1992 Table of Contents Introduction ...................................................................... 1 Background ...................................................................... 1 Regulations ...................................................................... 4 Vehicles Covered .................................................................. 5 Operations ........................................................................ 5 Wheelchair and Mobility Aid Standards ............................................... 5 Minimum Requirements ............................................................ 5 Periodic Revisions .................................................................. 6 How These Manuals are Organ ized ................................................... 6 Other Publications ................................................................. 7 §1192.1 Purpose. ................................................................... 8 §1192.2 Equivalent facilitation. ....................................................... 8 §1192.3 Defin itions. ............................................................... 9 §1192.4 Miscellaneous instructions. .................................................. 12 §1192.179 Trams, similar vehicles and systems. ........................................ 14 §1192.23 Mobility aid accessibility. .................................................. 15 (a) General (not ap plicable) (b) Vehicle lift. ............................................................ -

51694 Federal Register / Vol
51694 Federal Register / Vol. 63, No. 187 / Monday, September 28, 1998 / Rules and Regulations provided, and the number of times (b) Beginning October 30, 2006, the 5. Address: lllllllllllllll compensation was paid. It shall also Department will review the lllllllllllllllllllll include the name, address, telephone requirements of §§ 37.183, 37.185, 6. Phone number: llllllllllll number, and contact person name for 37.187, 37.191 and 37.193(a) and their 7. Date and time of trip: lllllllll 8. Location of need for equivalent service: the operator. implementation. The Department will lllllllllllllllllllll (c) Beginning October 30, 2000 for complete this review by October 29, 9. Was equivalent service provided for trip? large operators, and October 29, 2001 for 2007. Yesll noll small operators, and on that date in each (1) As part of this review, the 10. If the answer to items 9 and 10 is no, year thereafter, each fixed-route Department will consider factors attach documentation that compensation operator shall submit to the Department including, but not necessarily limited required by Department of a report on how many passengers with to, the following: Transportation regulations was paid. disabilities used the lift to board (i) The percentage of accessible buses [FR Doc. 98±25421 Filed 9±24±98; 2:15 pm] in the fixed-route fleets of large and accessible buses. For fixed-route BILLING CODE 4910±62±P operators, the report shall reflect small fixed-route operators. separately the data pertaining to 48-hour (ii) The success of small and large advance reservation service and other fixed-route operators' interim or ARCHITECTURAL AND service. -

Technical Aids*
Technical aids* E. PEIZER Veterans Administration Prosthetics Center, New York Introduction Wheelchairs The "disabled" represent a population with a Until recently relegation to a wheelchair was wide variety of functional losses. The VA are an admission of medical failure and so only the in complete accord with the idea that each most general features of the chair were of individual has a right to all the help technology concern. Now the wheelchair is looked upon as a can offer at a given time. The range of disability rehabilitation tool to replace functional loss. As and therefore the needs are enormous; few such, there must be judicious prescription of the centres, even in developed countries, have the most appropriate components from a vast time or the means to develop technical aids in number of options. sufficient quality and quantity to meet all these Figure 1 (left) shows a manually-propelled needs. It is essential to link all centres in the wheelchair which represents an archetype of a field to avoid costly duplication and repetition variety of such devices. Wheelchairs are specially of error, to advance development, and improve designated as standard, heavy duty, lightweight, treatment as rapidly as possible. Some links in amputee chairs, indoor chairs and, more this chain of co-operation have already been recently, outdoor chairs. Nevertheless all are forged; information about development and simply variations on this basic type with special evaluations are now being disseminated through applications based on moderate design changes such organizations as VA, ISPO and ICTA and a great deal of imagination. The Veterans (ISO). -

A SURVEY on TRADITIONAL METHODOLOGIES for PHYSICALLY CHALLENGED Dr
www.ijcrt.org © 2021 IJCRT | Volume 9, Issue 3 March 2021 | ISSN: 2320-2882 A SURVEY ON TRADITIONAL METHODOLOGIES FOR PHYSICALLY CHALLENGED Dr. Veena. S1 , Grace Varsha M2 , Malarvani V3,Swetha R4 1Professor, Department of Computer Science and Engineering 2 Student, Department of Computer Science and Engineering 3Student, Department of Computer Science and Engineering 4Student, Department of Computer Science and Engineering ,S. A Engineering College Independence of older adults.Fall incident with ABSTRACT: severe injuries occurs although various types of This study deals with the traditional walking devices are available.Mobility methodologies for physically challenged people. impairments prevents older adults from The survey on traditional methodologies describes performing their day to day activities which various traditional devices on mobility aids. This highly impacts on the quality of life.Mobility study also includes the necessities of mobility encompasses the ability of individual’s to move aids. It clearly explains the comparison and risks his or her body within an environment or between associated with mobility aids. It also says that environments also the ability to manipulate canes are the best mobility aid in traditional objects.The main objective of this research is to devices which are mostly used by old aged explore different options of traditional mobility people. assistive devices and to compare their pros and INTRODUCTION: cons.Mobility aids are the one which is designed to elevate the impact of mobility limitations -

The Benefits of Guide Dog Ownership L
Guide Dog Ownership The Benefits of Guide Dog Ownership L. Whitmarsh Guide Dogs, Hillfields, Burghfield, Reading RG7 3YG, UK Abstract There is an apparent discrepancy between the actual number of guide dog owners and the proportion of visually impaired people who might benefit from a guide dog. This research aims to provide an understanding of the reasons why many visually impaired people have not applied for a guide dog, the range of benefits offered by guide dogs, and how these might vary amongst different populations and under different circumstances. While previous research describes a number of psychological and social benefits of assistant animal ownership, consistent with the companion animal literature, it also points to the importance of personal and social context on the impact and effectiveness of assistance animals. The study described here involved a telephone survey of over 800 visually impaired people and found that independence, confidence, companionship, increased and changed social interaction, as well as increased mobility, are commonly-cited benefits of guide dog ownership. These psychological and social dimensions of owning a guide dog distinguish it from other mobility aids in its capacity to transform the lives of owners. However, as expected, demographic and contextual factors, such as gender, age, level of vision, and domestic circumstances, influence reasons for application and perceived benefits and drawbacks of guide dog ownership. The author argues that while this research has emphasised the tremendous impact a guide dog can have, providing the most appropriate mobility aid for an individual’s circumstances is the hallmark of effective rehabilitation service provision. The article also suggests ways in which perceived barriers to applying for a guide dog might be reduced. -

ADA Policy and Procedures
2014 ADA Policy Deviated Fixed Route Procedures Shirley Lyons Manager 807 E Main, P.O. Box 517 Molalla, OR 97038 Phone: 503-829-7000 Email: [email protected] South Clackamas Transportation District Americans with Disabilities Act of 1990 (ADA) ADA Policy and Deviated Fixed Route Procedures April 2014 Introduction and Purpose: This ADA policy is written to establish operating and service guidelines and procedures for the implementation of the requirements of the American with Disabilities Act of 1990 (ADA), the U.S. Department of Transportation regulations for implementing ADA (49 CFR Parts 27, 37 and 38), and applicable Oregon laws and regulations. The South Clackamas Transportation District SCTD Public Transit System operates three routes, two are commuter service routes and one operates on a deviated fixed route basis. SCTD complies with ADA requirements with respect to such services. Service Classifications: SCTD Routes: 1.) Molalla to Clackamas Community College- Commuter service 2.) Molalla to Canby- Commuter service 3.) Molalla City Bus- Deviated fixed route for the general public is a demand response service The Molalla City Bus Deviated Fixed Route is offered to all members of the public, not just persons with disabilities, therefore the service is considered “demand response” and SCTD is not required to provide ADA complementary paratransit. Policy Statement: It is the policy of South Clackamas Transportation District to comply with all the legal requirements of Federal and State laws and regulations as they pertain to individuals with disabilities. The transit system provides quality transportation services without discrimination to all persons including individuals with disabilities. Discrimination on the basis of disability against any person by transit system employees will not be condoned or tolerated.