The Human Nucleolus Organizer Regions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Splicing Factor XAB2 Interacts with ERCC1-XPF and XPG for RNA-Loop Processing During Mammalian Development
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.20.211441; this version posted July 21, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. The Splicing Factor XAB2 interacts with ERCC1-XPF and XPG for RNA-loop processing during mammalian development Evi Goulielmaki1*, Maria Tsekrekou1,2*, Nikos Batsiotos1,2, Mariana Ascensão-Ferreira3, Eleftheria Ledaki1, Kalliopi Stratigi1, Georgia Chatzinikolaou1, Pantelis Topalis1, Theodore Kosteas1, Janine Altmüller4, Jeroen A. Demmers5, Nuno L. Barbosa-Morais3, George A. Garinis1,2* 1. Institute of Molecular Biology and Biotechnology, Foundation for Research and Technology- Hellas, GR70013, Heraklion, Crete, Greece, 2. Department of Biology, University of Crete, Heraklion, Crete, Greece, 3. Instituto de Medicina Molecular João Lobo Antunes, Faculdade de Medicina da Universidade de Lisboa, Avenida Professor Egas Moniz, 1649-028 Lisboa, Portugal, 4. Cologne Center for Genomics (CCG), Institute for Genetics, University of Cologne, 50931, Cologne, Germany, 5. Proteomics Center, Netherlands Proteomics Center, and Department of Biochemistry, Erasmus University Medical Center, the Netherlands. Corresponding author: George A. Garinis ([email protected]) *: equally contributing authors bioRxiv preprint doi: https://doi.org/10.1101/2020.07.20.211441; this version posted July 21, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract RNA splicing, transcription and the DNA damage response are intriguingly linked in mammals but the underlying mechanisms remain poorly understood. Using an in vivo biotinylation tagging approach in mice, we show that the splicing factor XAB2 interacts with the core spliceosome and that it binds to spliceosomal U4 and U6 snRNAs and pre-mRNAs in developing livers. -

Nucleolus: a Central Hub for Nuclear Functions Olga Iarovaia, Elizaveta Minina, Eugene Sheval, Daria Onichtchouk, Svetlana Dokudovskaya, Sergey Razin, Yegor Vassetzky
Nucleolus: A Central Hub for Nuclear Functions Olga Iarovaia, Elizaveta Minina, Eugene Sheval, Daria Onichtchouk, Svetlana Dokudovskaya, Sergey Razin, Yegor Vassetzky To cite this version: Olga Iarovaia, Elizaveta Minina, Eugene Sheval, Daria Onichtchouk, Svetlana Dokudovskaya, et al.. Nucleolus: A Central Hub for Nuclear Functions. Trends in Cell Biology, Elsevier, 2019, 29 (8), pp.647-659. 10.1016/j.tcb.2019.04.003. hal-02322927 HAL Id: hal-02322927 https://hal.archives-ouvertes.fr/hal-02322927 Submitted on 18 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Nucleolus: A Central Hub for Nuclear Functions Olga Iarovaia, Elizaveta Minina, Eugene Sheval, Daria Onichtchouk, Svetlana Dokudovskaya, Sergey Razin, Yegor Vassetzky To cite this version: Olga Iarovaia, Elizaveta Minina, Eugene Sheval, Daria Onichtchouk, Svetlana Dokudovskaya, et al.. Nucleolus: A Central Hub for Nuclear Functions. Trends in Cell Biology, Elsevier, 2019, 29 (8), pp.647-659. 10.1016/j.tcb.2019.04.003. hal-02322927 HAL Id: hal-02322927 https://hal.archives-ouvertes.fr/hal-02322927 Submitted on 18 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. -

Unit 6-Nucleus
Unit 6-Nucleus Dr. Pallee shree Nucleus • Nucleus is the most important organelle in the cell • It distinguishes eukaryotic from prokaryotic cells • By housing the cell's genome, the nucleus serves both as the repository of genetic information and as the cell's control center • DNA replication, transcription, and RNA processing all take place within the nucleus Cont… • A nucleus is a double-membraned eukaryotic cell organelle that contains the genetic material. • It appears in an oval shape averages 5µm in width. • It often lies in the centre of a cell • The nucleus was the first organelle to be discovered • Nuclei 1st discovered and named by Robert Brown • Role of nucleus 1st demonestrated by Max Hammerling Ultra structure of Nucleus 1. Nuclear envelope 2. nuclear pores 3. Nucleoplasm 4. Nucleolus 5. Chromosomes 1. Structure of Nuclear envelope • The nuclear envelope has a complex structure consisting of a) Two nuclear membranes separated by a perinuclear space measuring about 20–40 nm across b) Underlying nuclear lamina • The nucleus is surrounded by a system of two concentric membranes, called the inner and outer nuclear membranes • The inner and outer nuclear membranes are joined at nuclear pore complexes a. Nuclear membranes • The outer nuclear membrane is continuous with the endoplasmic reticulum, so the space between the • The critical function of the inner and outer nuclear membranes nuclear membranes is to act as is directly connected with the lumen a barrier that separates the of the ER contents of the nucleus from the cytoplasm. • It is functionally similar to the membranes of the ER and has • Like other cell membranes, ribosomes bound to its cytoplasmic each nuclear membrane is a surface but protein composition phospholipid bilayer differ slightly as they are enriched in permeable only to small proteins which binds to cytoskeleton nonpolar molecules • The inner nuclear membrane carries • Other molecules are unable to proteins that are specific to the diffuse through the bilayer. -

Functional Proximity Mapping of RNA Binding Proteins Uncovers a Mitochondrial Mrna Anchor That Promotes Stress Recovery
Functional proximity mapping of RNA binding proteins uncovers a mitochondrial mRNA anchor that promotes stress recovery Wei Qin Stanford University Samuel Myers Broad Institute Dominique Carey The Broad Institute of MIT and Harvard Steven Carr Broad Institute Alice Ting ( [email protected] ) Stanford University https://orcid.org/0000-0002-8277-5226 Article Keywords: Unbiased Proteome Discovery, Nucleolus, Outer Mitochondrial Membrane, Local Translation, Functional Protein Mapping Posted Date: November 12th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-103196/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/49 Abstract Proximity labeling (PL) with genetically-targeted promiscuous enzymes has emerged as a powerful tool for unbiased proteome discovery. By combining the spatiotemporal specicity of PL with methods for functional protein enrichment, it should be possible to map specic protein subclasses within distinct compartments of living cells. Here we demonstrate this capability for RNA binding proteins (RBPs), by combining peroxidase-based PL with organic-aqueous phase separation of crosslinked protein-RNA complexes (“APEX-PS”). We validated APEX-PS by mapping nuclear RBPs, then applied it to uncover the RBPomes of two unpuriable subcompartments - the nucleolus and the outer mitochondrial membrane (OMM). At the OMM, we discovered the RBP SYNJ2BP, which retains specic nuclear-encoded mitochondrial mRNAs during translation stress, to promote their local translation and import of protein products into the mitochondrion during stress recovery. APEX-PS is a versatile tool for compartment- specic RBP discovery and expands the scope of PL to functional protein mapping. Introduction RNA-protein interactions are pervasive in both transient and stable macromolecular complexes underlying transcription, translation and stress response 1, 2. -
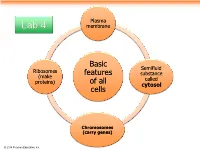
Basic Features of All Cells
Plasma Lab 4 membrane Basic Semifluid Ribosomes features substance (make called proteins) of all cytosol cells Chromosomes (carry genes) © 2014 Pearson Education, Inc. Prokaryotic cells are characterized by having . No nucleus . DNA in an unbound region called the nucleoid . No membrane-bound organelles . Cytoplasm bound by the plasma membrane © 2014 Pearson Education, Inc. Eukaryotic cells are characterized by having • DNA in a nucleus that is bounded by a membranous nuclear envelope • Membrane-bound organelles • Cytoplasm in the region between the plasma membrane and nucleus Eukaryotic cells are generally much larger than prokaryotic cells © 2014 Pearson Education, Inc. Figure 6.8a ENDOPLASMIC RETICULUM (ER) Nuclear envelope Rough ER Smooth ER Nucleolus NUCLEUS Flagellum Chromatin Centrosome Plasma membrane CYTOSKELETON: Microfilaments Intermediate filaments Microtubules Ribosomes Microvilli Golgi apparatus Peroxisome Lysosome Mitochondrion © 2014 Pearson Education, Inc. Figure 6.8b Nuclear envelope NUCLEUS Nucleolus Rough ER Chromatin Smooth ER Ribosomes Golgi Central vacuole apparatus Microfilaments CYTOSKELETON Microtubules Mitochondrion Peroxisome Plasma Chloroplast membrane Cell wall Plasmodesmata Wall of adjacent cell © 2014 Pearson Education, Inc. © 2014 Pearson Education, Inc. © 2014 Pearson Education, Inc. © 2014 Pearson Education, Inc. Table 6.1 © 2014 Pearson Education, Inc. Cell Walls of Plants The cell wall is an extracellular structure that distinguishes plant cells from animal cells Plant cell walls Prokaryotes, are made -

Driving Nucleolar Assembly
Downloaded from genesdev.cshlp.org on October 5, 2021 - Published by Cold Spring Harbor Laboratory Press PERSPECTIVE Driving nucleolar assembly Kathleen L. McCann,1 and Susan J. Baserga1,2,3,4 1Department of Genetics, 2Department of Molecular Biophysics and Biochemistry, 3Department of Therapeutic Radiology, Yale University School of Medicine, New Haven, Connecticut 06520, USA In this issue of Genes & Development,Grobandcol- breaks down (open mitosis), transcription of the ribosomal leagues (pp. 220–230) identify the minimal molecular re- RNA (rRNA) is inhibited, and the nucleolus disassem- quirements to assemble a fully functional nucleolus in bles. Upon completion of mitosis, rRNA transcription human cells and demonstrate the importance of the is reinitiated within the NOR, ribosome biogenesis fac- nucleolar transcription factor upstream binding factor tors are recruited, and the nucleolus is assembled. Only (UBF) as a mitotic bookmark at the ribosomal DNA NORs that are actively engaged in transcription can (rDNA). direct nucleolar assembly (Hernandez-Verdun 2011). Consequently, the nucleolus truly appears to be ‘‘an organelle formed by the act of building a ribosome’’ (Melese and Xue 1995). In all eukaryotes, the nucleolus is an essential, non- To begin to reveal the molecular mechanisms of nucle- membrane-bound organelle within the nucleus. Assem- olar formation, McStay’s laboratory (Mais et al. 2005; Grob bled around the ribosomal DNA (rDNA), the nucleolus et al. 2014) has applied synthetic biology. In an earlier is the site of ribosome biogenesis. In addition to its role report, they introduced 6.4 kb of DNA repeat sequences in making ribosomes, the nucleolus functions in other from the intergenic spacer of the Xenopus ribosomal gene important cellular processes, including stress sensing, into a noncanonical site in the human genome. -

Segregation of the Nucleolus Produced by Anthramycin1
[CANCER RESEARCH 28, 81-90, January 1968] Segregation of the Nucleolus Produced by Anthramycin1 Curtis Harris, Harold Grady, and Donald Svoboda Department oj Pathology, University oj Kansas Medical Center, Kansas City, Kansas 66103 SUMMARY mycin does not produce toxicity in the bone marrow and gastrointestinal tract (49). It possesses certain structural fea Anthramycin, an antibiotic possessing antitumor properties, tures in common with actinomycin D and the postulated biosyn- was administered to rats and mice in a single intraperitoneal thetic intermediates of the actinomycin^, 3-hydroxy-4-methyI- close ranging from 1 to 10 mg/kg body weight. Light micro anthraniloyl peptides (3,47). Like kanamycin, neomycin, and scopy showed that, at the highest dose, anthramycin produced other antibiotics (48), anthramycin permanently bleaches necrosis in the pancreas of rats but not in mice. By electron Euglena gracilis (11). microscopy, nucleolar changes were observed in the spleen and This report demonstrates that anthramycin is another agent kidney of both species by 1 hour. Acinar cells of the pancreas which causes nucleolar changes in pancreatic cells, reticulo showed nucleolar caps and segregation of nucleolar constituents endothelial cells of liver and spleen, and in proximal tubular in both species at 3 to 4 hours. By 10 hours segregation of the epithelium in the kidney. nucleolus and focal cytoplasmic degradation were prominent in pancreatic cells of rats given higher doses but were not MATERIALS AND METHODS observed in hepatocytes of either species. Anthramycin produced nucleolar alterations in Kupffer cells, Thirty-six inbred Fisher-344 male rats weighing between 95 endocrine and exocrine pancreatic cells, proximal tubular cells and 240 gm and fifteen C57 black mice weighing between 20 of the kidney, and in the reticuloendothelial cells of the spleen and 30 gm were used. -

Caspases Mediate Nucleoporin Cleavage, but Not Early Redistribution of Nuclear Transport Factors and Modulation of Nuclear Permeability in Apoptosis
Cell Death and Differentiation (2001) 8, 495 ± 505 ã 2001 Nature Publishing Group All rights reserved 1350-9047/01 $15.00 www.nature.com/cdd Caspases mediate nucleoporin cleavage, but not early redistribution of nuclear transport factors and modulation of nuclear permeability in apoptosis E Ferrando-May1, V Cordes2,3, I Biller-Ckovric1, J Mirkovic1, Val-Ala-aspartyl-¯uoromethylketone; DEVD-CHO, N-acetyl-Asp- DGoÈ rlich4 and P Nicotera*,5 Glu-Val-Asp-aldehyde 1 Chair of Molecular Toxicology, Department of Biology, University of Konstanz, 78457 Konstanz, Germany Introduction 2 Karolinska Institutet, Medical Nobel Institute, Department of Cellular and Molecular Biology, S-17177 Stockholm, Sweden The most evident morphological feature of apoptosis is the 3 Division of Cell Biology, Germany Cancer Research Center, D-69120, disassembly of the nucleus, which involves the condensation Heidelberg, Germany 4 of chromatin and its segregation into membrane-enclosed Zentrum fuÈr Molekulare Biologie der UniversitaÈt Heidelberg, D-69120, 1 Heidelberg, Germany particles. Biochemical hallmarks of apoptotic nuclear 5 MRC Toxicology Unit, Hodgkin Building, University of Leicester, Lancaster execution are DNA cleavage in large and small (oligonu- Road, Leicester LE1 9HN, UK cleosomal-sized) fragments, as well as the specific proteo- * Corresponding author: P Nicotera, MRC Toxicology Unit, Hodgkin Building, lysis of several nuclear substrates. Major effectors of University of Leicester, Lancaster Road, Leicester LE1 9HN, UK. apoptotic nuclear changes are members of the cysteine Tel +44-116-2525611; Fax: +44-116-2525616; E-mail: [email protected] protease family of caspases. Nuclear substrates for caspases 2,3 Received 23.11.00; revised 22.12.00; accepted 29.12.00 include nucleoskeletal elements like lamins, and proteins Edited by M Piacentini involved in the organisation and replication of DNA, like SAF- A, MCM3 and RCF140.4±6 Cleavage of nuclear proteins may have important Abstract implications for the apoptotic process. -

The Nucleolus As a Multiphase Liquid Condensate
REVIEWS The nucleolus as a multiphase liquid condensate Denis L. J. Lafontaine 1 ✉ , Joshua A. Riback 2, Rümeyza Bascetin 1 and Clifford P. Brangwynne 2,3 ✉ Abstract | The nucleolus is the most prominent nuclear body and serves a fundamentally important biological role as a site of ribonucleoprotein particle assembly, primarily dedicated to ribosome biogenesis. Despite being one of the first intracellular structures visualized historically, the biophysical rules governing its assembly and function are only starting to become clear. Recent studies have provided increasing support for the concept that the nucleolus represents a multilayered biomolecular condensate, whose formation by liquid–liquid phase separation (LLPS) facilitates the initial steps of ribosome biogenesis and other functions. Here, we review these biophysical insights in the context of the molecular and cell biology of the nucleolus. We discuss how nucleolar function is linked to its organization as a multiphase condensate and how dysregulation of this organization could provide insights into still poorly understood aspects of nucleolus-associated diseases, including cancer, ribosomopathies and neurodegeneration as well as ageing. We suggest that the LLPS model provides the starting point for a unifying quantitative framework for the assembly, structural maintenance and function of the nucleolus, with implications for gene regulation and ribonucleoprotein particle assembly throughout the nucleus. The LLPS concept is also likely useful in designing new therapeutic strategies to target nucleolar dysfunction. Protein trans-acting factors Among numerous microscopically visible nuclear sub- at the inner core where rRNA transcription occurs and Proteins important for structures, the nucleolus is the most prominent and proceeding towards the periphery (Fig. -

Clusters of Bacterial RNA Polymerase Are Biomolecular Condensates That Assemble Through Liquid–Liquid Phase Separation
Clusters of bacterial RNA polymerase are biomolecular condensates that assemble through liquid–liquid phase separation Anne-Marie Ladouceura,1, Baljyot Singh Parmarb,1, Stefan Biedzinskia, James Walla, S. Graydon Topea, David Cohna, Albright Kima, Nicolas Soubrya, Rodrigo Reyes-Lamothea, and Stephanie C. Webera,b,2 aDepartment of Biology, McGill University, Montreal, QC H3A 1B1, Canada; and bDepartment of Physics, McGill University, Montreal, QC H3A 2T8, Canada Edited by Richard A. Young, Massachusetts Institute of Technology, Cambridge, MA, and approved June 23, 2020 (received for review March 17, 2020) Once described as mere “bags of enzymes,” bacterial cells are in possibility that prokaryotes also use LLPS to compartmentalize fact highly organized, with many macromolecules exhibiting non- their cells. uniform localization patterns. Yet the physical and biochemical In eukaryotes, LLPS is mediated by proteins containing mul- mechanisms that govern this spatial heterogeneity remain largely tivalent domains and disordered regions (22–24) that bring unknown. Here, we identify liquid–liquid phase separation (LLPS) as molecules together into dynamic condensates through transient a mechanism for organizing clusters of RNA polymerase (RNAP) in interactions. Sequence analysis predicts that bacterial proteomes Escherichia coli . Using fluorescence imaging, we show that RNAP have a much lower frequency of disordered regions than quickly transitions from a dispersed to clustered localization pattern eukaryotic proteomes: Only 4.2% compared to 33%, respectively as cells enter log phase in nutrient-rich media. RNAP clusters are (25). This paucity of disorder raises doubts about the prevalence sensitive to hexanediol, a chemical that dissolves liquid-like com- of LLPS in this domain of life. -

THE NUCLEOLUS ORGANIZER REGION of MAIZE (ZEA MAYS L.): TESTS for RIBOSOMAL GENE COMPENSATION OR MAGNIFICATION1~Z
THE NUCLEOLUS ORGANIZER REGION OF MAIZE (ZEA MAYS L.): TESTS FOR RIBOSOMAL GENE COMPENSATION OR MAGNIFICATION1~z R. L. PHILLIPS, D. F. WEBERS, R. A. KLEESE4 AND S. S. WANG Department of Agronomy and Plant Genetics, University of Minnesota, Si. Paul, Minnesota 55101 Department of Biological Sciences, Illinois State University, Normal, Illinois 61761 Manuscript received December 6, 1973 ABSTRACT Ribosomal gene compensation and magnification that might be detected on a whole-plant basis was not found in maize. Plants monosomic for chromo- some 6 (the NOR chromosome) were compared with monosomic-8 and mono- somic-IO plants, disomic sibs, and parental lines. Assuming no rDNA com- pensation, monosomic-6 plants showed approximately the decrease expected in rRNA cistron number. Monosomic-8 had a normal ribosomal gene number, while monosomic-IO showed a decrease; but further documentation is needed. Besides demonstrating the absence of gene compensation, the results document our previous conclusion that maize chromosome 6 carries DNA complementary to ribosomal RNA. Further documentation was provided from studies with trisomic chromosome 6 plants showing proportional increases in ribosomal gene number. Progeny of the monosomic plants crossed as males to a standard singlecross hybrid possessed expected ribosomal gene numbers suggesting the lack of ribosomal gene magnification.-The ragged (rgd) mutant of maize, suspected of being deficient in rRNA cistrons, had a normal number. AMPLIFICATION of genes coding for ribosomal RNA occurs widely in the oocyte nuclei of animal species. Presumably the tremendous requirements for rRNA during early embryogenesis necessitates a mechanism for the rapid synthesis of large quantities of rRNA. In some cases rRNA is furnished by the nurse cells while the nucleus of the oocyte is relatively inactive (GALL1969). -

Centriole Lysosomes Chloroplasts Mitochondrion Endoplasmic Reticulum (ER) Smooth ER Cell Membrane Nucleolus Golgi Body
Virtual Cell Worksheet- ANSWER KEY 1. Centrioles are only found in animal cells. They function in cell division . They have 9 groups of 3 Centriole arrangement of the protein fibers. Draw a picture of a centriole in the box. 2. Lysosomes are called suicide sacks. They are produced by the golgi body. They consist of a single Lysosomes membrane surrounding powerful digestive enzymes. Those lumpy brown structures are digestive enzymes . They help protect you by destroying the bacteria that your white blood cells engulf. Lysosomes act as a clean up crew for the cell. Zoom in and draw what you see. 3. Chloroplasts are the site of photosynthesis . They consist of a double membrane. The stacks of disk Chloroplasts like structures are called the grana . The membranes connecting them are the thylakoid membranes. Zoom in and draw a picture. 4. Mitochondrion is the powerhouse of the cell. It is the site of respiration . It has a double membrane. Mitochondrion The inner membrane is where most aerobic respiration occurs. The inner membrane is ruffled with a very large surface area. These ruffles are called cristae . Mitochondria have their own DNA and manufacture some of their own proteins . Draw a picture of the mitochondrion with its membrane cut. 5. Endoplasmic Reticulum (ER) is a series of double membranes that loop back and forth between the Endoplasmic cell membrane and the nucleus . These membranes fill the cytoplasm but you cannot see them because Reticulum (ER) they are very transparent . The rough E.R. has ribosomes attached to it. This gives it its texture.