Dietary Conjugated Linoleic Acid-Enriched Cheeses Influence
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Great Food, Great Stories from Korea
GREAT FOOD, GREAT STORIE FOOD, GREAT GREAT A Tableau of a Diamond Wedding Anniversary GOVERNMENT PUBLICATIONS This is a picture of an older couple from the 18th century repeating their wedding ceremony in celebration of their 60th anniversary. REGISTRATION NUMBER This painting vividly depicts a tableau in which their children offer up 11-1541000-001295-01 a cup of drink, wishing them health and longevity. The authorship of the painting is unknown, and the painting is currently housed in the National Museum of Korea. Designed to help foreigners understand Korean cuisine more easily and with greater accuracy, our <Korean Menu Guide> contains information on 154 Korean dishes in 10 languages. S <Korean Restaurant Guide 2011-Tokyo> introduces 34 excellent F Korean restaurants in the Greater Tokyo Area. ROM KOREA GREAT FOOD, GREAT STORIES FROM KOREA The Korean Food Foundation is a specialized GREAT FOOD, GREAT STORIES private organization that searches for new This book tells the many stories of Korean food, the rich flavors that have evolved generation dishes and conducts research on Korean cuisine after generation, meal after meal, for over several millennia on the Korean peninsula. in order to introduce Korean food and culinary A single dish usually leads to the creation of another through the expansion of time and space, FROM KOREA culture to the world, and support related making it impossible to count the exact number of dishes in the Korean cuisine. So, for this content development and marketing. <Korean Restaurant Guide 2011-Western Europe> (5 volumes in total) book, we have only included a selection of a hundred or so of the most representative. -

The Diversity of Fatty Acid Composition in Traditional and Rare Oil Crops Cultivated in Russia
REVIEW COMMUNICATIONS PLANT SCIENCE The diversity of fatty acid composition in traditional and rare oil crops cultivated in Russia Vera Gavrilova, Tatyana Shelenga, Elizaveta Porokhovinova, Aleksandra Dubovskaya, Nina Kon’kova, Sergey Grigoryev, Larisa Podolnaya, Aleksey Konarev, Tamara Yakusheva, Natalya Kishlyan, Andrey Pavlov, and Nina Brutch Federal Research Center N. I. Vavilov All-Russian Institute of Plant Genetic Resources, Bol’shaya Morskaya ul., 42–44, Saint Petersburg, 190000, Russian Federation Address correspondence and requests for materials to Nina Brutch, [email protected] Abstract This review is devoted to the description of chemical peculiarities of industrial oil crops cultivated (or prospective for cultivation) in Russia, which are stored in the VIR collection. Different crops have similar fatty acids biosynthesis path- ways, but each species has its own individualities in the chemical composition of the oil and its genetic control. The diversity of oil crop chemical composition Citation: Gavrilova, V., Shelenga, T., Porokhovinova, E., Dubovskaya, A., opens the possibility of its multipurpose utilization practically in all industrial Kon’kova, N., Grigoryev, S., Podolnaya, L., segments. Sunflower, rapeseed, flax, mustard, camelina and safflower are cul- Konarev, A., Yakusheva, T., Kishlyan, N., Pavlov, A., and Brutch, N. 2020. The diversity tivated in Russia as oil crops. Castor beans, perilla, lallemantia and noog are of fatty acid composition in traditional not cultivated on an industrial scale, but have original oil properties and are and rare oil crops cultivated in Russia. Bio. Comm. 65(1): 68–81. https://doi. prospective for future cultivation. Hemp and poppy seeds contain oil valuable org/10.21638/spbu03.2020.106 for food, but they are not widespread. -

The Wellness Family
The Wellness Family Dr. Schroeder Keeps You Informed This particular plant oil is one of the few cooking oils that Cooking Oils contains about 75% of its fat as oleic acid (a monounsat- urated, omega-9 fatty acid). If you have typically been using You’ve taken the time to buy really fresh vegetables, to corn oil or vegetable oil, research has shown that altering dice them up into a delicious mix so that you and your your diet by replacing that choice with olive oil may lead to family can eat healthier and feel better. However, the a significant decrease in your total blood cholesterol and an decision of what oil to cook them in is equally important. improved LDL:HDL ratio. The wrong cooking oil can turn a healthy mix of fresh vegetables into an unhealthy mix of mush. Olive oil does have one downside and that is its tendency to degrade in a very short amount of time, especially if All About Oils stored in a warm place (like a kitchen). In just a month or so, There are several different choices of oils depending stored olive oil will begin to break-down and eventually go upon whether you’re baking, cooking, marinating, rancid. Food cooked with rancid olive oil will have a bad flavoring or garnishing. The right choice is going to depend on the purpose: i.e. the food you are preparing When preparing healthy and how it’s being prepared. foods for your family When baking, your healthiest choices include coconut, remember: the oil you palm and more, but high oleic safflower and sunflower oil are the best for this purpose. -

Agenda Item 7(B) CX/PR 18/50/7-Add.1 April 2018 JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE on PESTICIDE RESIDUES 50Th Session Haikou, PR
E Agenda Item 7(b) CX/PR 18/50/7-Add.1 April 2018 JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON PESTICIDE RESIDUES 50th Session Haikou, PR. China, 9 - 14 April 2018 Comments at Agenda Item 7(b) submitted by Canada, China, Egypt, European Union, Kenya, Paraguay and Turkey REVISION OF THE CLASSIFICATION OF FOOD AND FEED: CLASS A: PRIMARY FOOD COMMODITIES OF PLANT ORIGIN TYPE 05: HERBS AND SPICES GROUP 027 HERBS GROUP 028 SPICES Canada BACKGROUND Groups 027 and 028 were finalized by CCPR43 in 2011 and were retained at Step 7 pending finalization of the revision of the Classification. CCPR44 (2012) agreed to hold the commodity group on “edible flowers” at Step 7 pending finalization of the revision of the Classification in relation to the herbs group. CURRENT STATUS Subsequent to CCPR43 and CCPR44, the EWG revised Group 027 and Group 028 to consider new commodities in accordance with the terms of reference given by CCPR49 (2017). As a result, the EWG has proposed the following changes: Additional commodities added to both Group 027 and Group 028. Wasabi stem was moved to Group 027 from Group 028 as it is classified as an herb. Caraway seed was moved from 028A to 028B. Cross references were added for black, brown and white mustard seeds. Kokam was removed as it is already a member Group 006 (Assorted tropical and sub-tropical fruits – inedible peel). The EU proposed that Subgroups 028H Citrus Peel and 028I Dried Chili Peppers would be more appropriate for Class D. Processed Foods of Plant Origin. -

Dict-En-Fr-Food V3
Dictionnaire Anglais-Français de l’Alimentation English-French Food Dictionary Version 3.0 Pascal Médeville (CC BY-NC-ND 4.0) 1 This work is distributed under Creative Commons license CC BY-NC-ND 4.0. Please revise the information below and stay within the limits of the license. 2 Le présent travail est distribué dans le cadre de la licence Creative Commons CC BY-NC-ND 4.0. Veuillez lire les informations ci-dessous et respecter les limites imposées. 3 Sigles et abréviations utilisés dans ce dictionnaire : adj. : adjectif agr. : agriculture bot. : botanique ichtyol. : ichtyologie Ind. : Indonésie it. : Italie J : Japon microbiol. : microbiologie œnol. : œnologie p.ex. : par exemple UK : Royaume Uni US : États-Unis d’Amérique vét. : médecine vétérinaire zool. : zoologie Historique des versions/Version history : Date Objet No. de version Déc. 2008 Version initiale 1.0 Juin 2009 Corrections diverses ; Ajout de nouvelles sources ; Ajout de nouvelles 2.0 références bibliographiques Avril 2020 Ajout de nouvelles entrées ; Corrections diverses ; Ajout de nouvelles 3.0 sources ; Ajout de nouvelles references bibliographiques 4 Pour vos traductions anglais-français ou chinois-français dans le domaine de la gastronomie ou de l’agro-alimentaire, veuillez prendre contact avec Pascal Médeville à l’une des adresses suivantes : [email protected] ou [email protected]. For all your food and agrifood translation needs (English to French of Chinese to French), you can contact Pascal Médeville, at [email protected] or [email protected]. Pour toutes vos traductions et besoins de publication assistée par ordinateur en chinois ou d’autres langues asiatiques, n’hésitez pas à faire appel à Pascal Médeville ou à Parallels Translation Office : www.parallels-translation.net. -

The Energy Paradox by Dr
The Energy Paradox By Dr. Steven Gundry Yes and No Foods for Energy The “Yes” List Cruciferous Vegetables • arugula • bok choy • broccoli • Brussels sprouts Other Vegetables • cabbage (green and red) • artichokes • cauliflower • asparagus • collards • bamboo shoots • kale • beets (raw) • kimchi • carrot greens • kohlrabi • carrots (raw) • napa cabbage • celery • radicchio • chicory • sauerkraut (raw) • chives • Swiss chard • daikon radish • watercress • fiddlehead ferns • garlic Leafy Greens • garlic scapes • algae • ginger • basil • hearts of palm • butter lettuce • horseradish • cilantro • Jerusalem artichokes (sunchokes) • dandelion greens • leeks • endive • lemongrass • escarole • mushrooms • fennel • nopales (cactus; available online) • mesclun (baby greens) • okra • mint • onions • mizuna • parsnips • mustard greens • puntarella • parsley • radishes • perilla • rutabaga • purslane • scallions • red and green leaf lettuces • shallots • romaine lettuce • water chestnuts • sea vegetables • seaweed Fruits That Act like Fats • spinach • Avocado (up to a whole one per day) • Olives, all types 1 THE ENERGY PARADOX | YES LIST Energy Bars (limit to one per day, please) • Adapt Bars: Coconut and Chocolate • B-Up (made by Yup): Chocolate Mint, Chocolate Chip Cookie Dough, Sugar Cookie • GundryMD Bars • Keto Bars: Almond Butter Brownie, Salted Caramel, Lemon Poppyseed, Chocolate Chip Cookie Dough Oils • MariGold Bars: ChocoNut, Pure Joy, Espresso, • algae oil (Thrive culinary brand) Ginger Coconut • avocado oil • Primal Kitchen Bars: Almond -

E6e93905d5b07240c02ac30ab9
Vol. 10(12) pp. 352-358, December 2016 DOI: 10.5897/AJFS2016.1500 Article Number: EF34CC861434 ISSN 1996-0794 African Journal of Food Science Copyright © 2016 Author(s) retain the copyright of this article http://www.academicjournals.org/AJFS Full Length Research Paper Physicochemical and sensory characteristics of pepper oil sauce prepared from perilla oil Hye-Ryun Kim1, Sanjeev Kumar Dhungana2, Il-Doo Kim3 and In-Joo Park1* 1Cheonnyeonmiin Co. Ltd., Gyeongju-si 38180, Korea. 2School of Applied Biosciences, Kyungpook National University, Daegu 41566, Korea. 3International Institute of Agricultural Research and Development, Kyungpook National University, Daegu 41566, Korea. Received 15 August, 2016, Accepted 21 September, 2016 Pepper oil sauce or ‘rayu’, is one of the most famous traditional condiments in Korea, Japan and China. Generally, it is prepared using edible oil from soybean, corn among others. Since perilla possesses high amount of omega-3 polyunsaturated fatty acids, the objective of the present study was to investigate the quality characteristics of pepper oil sauces prepared using perilla oil. The quality characteristics of perilla sauces (ONP: onion pepper oil, OIP: oil cake pepper oil and SHP: shrimp pepper oil) were compared with those of the commercially available sauce in Korea (KPO). Carbohydrate and sugar contents and calorie and peroxide values of commercial sauce were higher than those of the perilla oil sauces. However, crude protein and fat were higher in the perilla oil sauces. Soluble solid content of perilla oil sauces was significantly high as compared to that of KPO. The sauce prepared from perilla oil was found to be 12 times higher in omega-3 polyunsaturated fatty acid and 25%less in sodium than a commercial pepper oil sauce. -

Oxidized Perilla and Linseed Oils Induce Neuronal Apoptosis by Caspase-Dependent and -Independent Pathways
foods Article Oxidized Perilla and Linseed Oils Induce Neuronal Apoptosis by Caspase-Dependent and -Independent Pathways 1, , 2, 1 1 1 Yuki Ueno y *, Yoshiyuki Kawamoto y, Yamato Nakane , Risa Natsume , Kyoko Miura , Yui Okumura 2, Takashi Murate 2, Emi Hattori 1 and Toshihiko Osawa 1 1 Department of Health and Nutrition, Faculty of Psychological and Physical Science, Aichi Gakuin University, 12 Araike Iwasaki-cho, Nisshin, Aichi 470-0195, Japan 2 Department of Biomedical Sciences, Collage of Life and Health Sciences, Chubu University, Kasugai, Aichi 487-8501, Japan * Correspondence: [email protected]; Tel.: +81-561-73-1111 These authors equally contribute to this work. y Received: 17 March 2020; Accepted: 22 April 2020; Published: 26 April 2020 Abstract: Alpha-linolenic acid (ALA), a polyunsaturated fatty acid, is involved in bioregulatory functions. In recent years, the health-promoting effects of vegetable-derived edible oils rich in ALA have attracted attention. ALA has a variety of physiological effects such as anti-arteriosclerotic and antiallergic properties, but is prone to oxidation. Therefore, safety concerns exist with regard to adverse effects on humans induced by its oxides. However, the effects on neuronal cells induced by oxidized ALA-rich oils, such as perilla and linseed oils, have not been fully investigated. This information is very important from the viewpoint of food safety. In this study, we investigated the effects of oxidized perilla and linseed oils, which are rich in ALA, on the toxicity of neuronal SH-SY5Y cells. Perilla and linseed oils were significantly oxidized compared with other edible vegetable oils. These oxidized oils induce neuronal cell death and apoptosis via caspase-dependent and -independent pathways through reactive oxygen species (ROS) generation. -
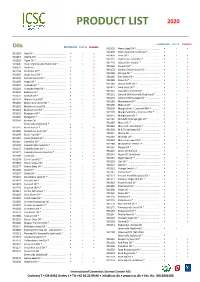
Product List 2020
PRODUCT LIST 2020 CONVENTIONAL ORGANIC STABILIZED Oils CONVENTIONAL ORGANIC STABILIZED 901199 Hemp Seed Oil * .................................. • • • 901499 Hemp Seed Oil Unrefined * ................. • • 901193 Acai Oil * ............................................. • • • 901450 Inchi Oil *............................................. • • • 901367 Alfalfa Oil* ........................................... • • • 901112 Jojoba Oil – Colorless * ........................ • • • 901228 Algae Oil * ........................................... • • • 901110 Jojoba Oil – Golden * ........................... • • • 907440 Aloe Oil (Internally Stabilized)* .......... • • 901162 Kakadu Oil * ........................................ • • • 906221 Amla Oil .............................................. • • 901152 Kalahari Melon Seed Oil * ................... • • • 901148 Andiroba Oil * ..................................... • • • 901168 Karanja Oil * ........................................ • • • 901387 Apple Seed Oil * .................................. • • • 901165 Kiwi Seed Oil * ..................................... • • • 901176 Apricot Kernel Oil * ............................. • • • 901185 Kukui Oil * ........................................... • • • 901195 Argan Oil * ........................................... • • • 901180 Lemon Seed Oil * ................................ • • • 901118 Avocado Oil * ...................................... • • • 901421 Lime Seed Oil * .................................... • • • 901218 Avocado Seed Oil * ............................. -

(L.) Britt 2 3 Hiwa M. Ahmed1,*
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 22 October 2018 doi:10.20944/preprints201810.0487.v1 Peer-reviewed version available at Molecules 2018, 24, 102; doi:10.3390/molecules24010102 1 Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla 2 frutescens (L.) Britt 3 4 Hiwa M. Ahmed1,* 5 6 1Sulaimani Polytechnic University, Slemani, Kurdistan Regional Government/Iraq 7 8 *Correspondence 9 Hiwa M. Ahmed 10 [email protected], [email protected] 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 1 © 2018 by the author(s). Distributed under a Creative Commons CC BY license. Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 22 October 2018 doi:10.20944/preprints201810.0487.v1 Peer-reviewed version available at Molecules 2018, 24, 102; doi:10.3390/molecules24010102 51 Abstract 52 Perilla frutescens (L.) Britt. (PF) is an annual herbal medicinal, aromatic, functional food and 53 ornamental plant that belongs to the mint family, Lamiaceae. The origin of perilla traces back 54 to East Asian countries (China, Japan, Korea, Taiwan, Vietnam and India), where it has been 55 used as a valuable source of culinary and traditional medicinal uses. Leaves, seeds and stems 56 of P. frutescens are used for various therapeutic applications in folk medicine. In the absence 57 of comprehensive review regarding all aspects of perilla, thus this review aims to present an 58 overview pertaining to the botanical drug, ethnobotany, phytochemistry and biological 59 activity. -

Oil and Fats Anise Seed Oil Apricot Kernel Oil Avellana Oil Avocado Oil
Oil and Fats Anise seed oil Apricot kernel oil Avellana oil Avocado oil Baobab oil Chia seed oil Cranberry seed oil Strawberry seed oils Tigernuts oil (earth almond oil) Peanut oil Fig seed oil Goji seed oil Pomegranate seed oil Cucumber seed oil Rose hip seed oil Hemp seed oil Hazelnut oil Raspberry seed oil Elderberry seed oil Black currant seed oil Jojoba oil Coffee bean oil Prickly pear seed oil Camellia oleifera seed oil Cherry kernel oil Coconut oil Coriander seed oil Cumin seed oil Pumpkin seed oil Camelina seed oil Linseed oil Almond oil Poppy seed oil Moringa oil Evening primrose oil Neem oil Brazil nut oil Passion fruit seed oil Pequi oil Perilla seed oil Peach kernel oil Plum kernel oil Castor oil Sacha inchi seed oil Sea buckthorn pulp oil Sea buckthorn seed oil Black cumin seed oil Tamanu oil Grape seed oil Brazil nut oil Walnut oil Watermelon seed oil Wheat germ oil Cedar nut oil Açai Oil Ahiflower Oil Amaranth Oil Argan Oil Avocado Oil Babassu Oil Black Currant Oil Borageseed Oil Brazilnut Oil Broccoliseed Oil Calendula Oil Camelina Oil Castor Oil Chilean Hazelnut Oil Coconut Oil Corn Oil Cottonseed Oil Evening Primrose Oil Grapeseed Oil Groundnut Oil Hazelnut Oil Hemp Oil Kukuinut Oil Laurel Oil Linseed Oil Macadamianut Oil Manketti Oil Marula Oil MCT Oil Meadowfoam Seed Oil Milk Thistle Oil Moringa Oil Mustardseed Oil Olive Oil Olus Oil Omega-3-6-9-Oils Palm Oil Palmkernel Oil Palmolein Palmstearin Oil Passionfruit Seed Pecan Nut Oil Perilla Oil Pistachionut Oil Pomegranate Oil Poppyseed Oil Pumpkinseed Oil Rapeseed Oil Rice Bran Oil Rose Hip Oil Sacha Inchi Oil Safflower Oil Seabuckthorn Pulp Oil Sesame Oil Soybean Oil St. -

The Plant Paradox Quick and Easy
The Plant Paradox Quick and Easy The 30-Day Plan to Lose Weight, Feel Great, and Live Lectin- Free Steven R. Gundry This book contains advice and information relating to health care. It should be used to supplement rather than replace the advice of your doctor or another trained health professional. If you know or suspect you have a health problem, it is recommended that you seek your physician’s advice before embarking on any medical program or treatment. All efforts have been made to assure the accuracy of the information contained in this book as of the date of publication. This publisher and the author disclaim liability for any medical outcomes that may occur as a result of applying the methods suggested in this book. the plant paradox quick and easy. Copyright © 2019 by Steven R. Gundry. All rights reserved. Printed in the United States of America. No part of this book may be used or reproduced in any manner whatsoever without written permission except in the case of brief quotations embodied in critical articles and reviews. For information, address HarperCollins Publishers, 195 Broadway, New York, NY 10007. HarperCollins books may be purchased for educational, business, or sales promotional use. For information, please email the Special Markets Department at [email protected]. first edition Library of Congress Cataloging- in- Publication Data has been applied for. ISBN 978-0-06-291199-5 (pbk.) 19 20 21 22 23 lsc 10 9 8 7 6 5 4 3 2 1 1 2 Once our bodies are able to finally get off the roller coaster of exposure to a damaging diet and deadly disruptors, it is amazing how beautifully they can heal themselves.