Cultural Control Methods That Effect the Development and Spread of Corynespora Cassiicola (Berk
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ekspedisi Saintifik Biodiversiti Hutan Paya Gambut Selangor Utara 28 November 2013 Hotel Quality, Shah Alam SELANGOR D
Prosiding Ekspedisi Saintifik Biodiversiti Hutan Paya Gambut Selangor Utara 28 November 2013 Hotel Quality, Shah Alam SELANGOR D. E. Seminar Ekspedisi Saintifik Biodiversiti Hutan Paya Gambut Selangor Utara 2013 Dianjurkan oleh Jabatan Perhutanan Semenanjung Malaysia Jabatan Perhutanan Negeri Selangor Malaysian Nature Society Ditaja oleh ASEAN Peatland Forest Programme (APFP) Dengan Kerjasama Kementerian Sumber Asli and Alam Sekitar (NRE) Jabatan Perlindungan Hidupan Liar dan Taman Negara (PERHILITAN) Semenanjung Malaysia PROSIDING 1 SEMINAR EKSPEDISI SAINTIFIK BIODIVERSITI HUTAN PAYA GAMBUT SELANGOR UTARA 2013 ISI KANDUNGAN PENGENALAN North Selangor Peat Swamp Forest .................................................................................................. 2 North Selangor Peat Swamp Forest Scientific Biodiversity Expedition 2013...................................... 3 ATURCARA SEMINAR ........................................................................................................................... 5 KERTAS PERBENTANGAN The Socio-Economic Survey on Importance of Peat Swamp Forest Ecosystem to Local Communities Adjacent to Raja Musa Forest Reserve ........................................................................................ 9 Assessment of North Selangor Peat Swamp Forest for Forest Tourism ........................................... 34 Developing a Preliminary Checklist of Birds at NSPSF ..................................................................... 41 The Southern Pied Hornbill of Sungai Panjang, Sabak -

Evidence That the Biocontrol Agent Bacillus Cereus Synthesizes Protein That Can Elicit Increased Resistance of Tomato Leaves to Corynespora Cassiicola
Tropical Plant Pathology, vol. 35, 1, 011-015 (2010) Copyright by the Brazilian Phytopathological Society. Printed in Brazil www.sbfito.com.br RESEARCH ARTICLE / ARTIGO Evidence that the biocontrol agent Bacillus cereus synthesizes protein that can elicit increased resistance of tomato leaves to Corynespora cassiicola Reginaldo S. Romeiro1, Roberto Lanna Filho1, Dirceu Macagnan2, Flávio A.O. Garcia1 & Harllen S.A. Silva3 1Departamento de Fitopatologia, Universidade Federal de Viçosa, 36570-000 Viçosa, MG, Brazil; 2Centro Federal de Educação Tecnológica de Rio Verde, 75901-970, Rio Verde, GO, Brazil; 3Embrapa Mandioca e Fruticultura, 44380-000, Cruz das Almas, BA, Brazil Author for correspondence: Dirceu Macagnan, e-mail: [email protected] ABSTRACT Isolate UFV-101 of Bacillus cereus was selected in previous studies for promoting growth inducing resistance in plants. In a previous study, supernatant from cultures of the microorganism in a liquid medium was found to induce resistance in tomato foliage against the pathogens Pseudomonas syringae pv. tomato, Xanthomonas vesicatoria, Alternaria solani and Corynespora cassiicola. In the present work the microorganism was grown in a minimal medium for 48 h and the cells precipitated for centrifugation. The supernatant was concentrated by lyophilization, dialyzed in a 12 kDa cut-off point membrane and fractioned in column containing Sephadex G25 balanced in PBS buffer. The fractions corresponding to a protein peak were applied to tomato seedlings. After four days leaflets were collected and inoculated with the pathogen. C. cassiicola. The numbers of lesions produced by the pathogen on leaflets exposed to the bacterial supernatant were similar to those exposed to acibenzolar-S-methyl but fewer than in those treated with water. -

Aeschynanthus Pulcher Lipstick Plant LIPSTICK PLANT
The Gardener’s Resource 435 W. Glenside Ave. Since 1943 Glenside, PA 19038 215-887-7500 Aeschynanthus pulcher Lipstick Plant LIPSTICK PLANT Lipstick plants are easy indoor flowering houseplants that when given the right amount of light and water, they produce numerous red or orange small tubular flowers that resemble a tube of lipstick. Not only are the flowers colorful, the leaves can be light green, dark green or green and maroon. Light: The Lipstick vine will not bloom without adequate light. They require very bright, indirect light. Avoid placing this plant in full shade or full sun. Direct sun will burn the leaves. The plant needs bright light for a portion of the day, but not all day long. Water: If you allow the top 25% of the soil to dry out before watering, this plant will flower more frequently and more abundant. If the leaves appear soft and shriveled, give it more water. These plants will also lose green leaves TIPS: when over-watered. • Lipstick plants like warm temperatures between 75-85°F. : Fertilizer every other week in the Fertilizing • Prefers high humidity, but will do well in Spring and Summer and only monthly in the basic household humidity too. Fall and Winter with a houseplant food high in • Trim the long vines to prevent the plant phosphorous. Always dilute the fertilizer to ½ from becoming thin and straggly. the recommended strength. • Lipstick Plants are Non-Poisonous. • Keep a lipstick plant in a small pot will help it produce more flowers. When put into a large container, instead of producing flowers it grows more leaves. -

Redalyc.Antagonistic Propierties of Micromycetes Isolated From
Revista Mexicana de Micología ISSN: 0187-3180 [email protected] Sociedad Mexicana de Micología México Moreno-Pérez, Pablo; Gamboa-Angulo, Marcela; Heredia, Gabriela; Canto-Canché, Blondy; Rosado-Vallado, Miguel; Medina-Baizabal, Irma L.; Tapia-Tusell, Raúl Antagonistic propierties of micromycetes isolated from sinkholes of the Yucatán Península against fungal phytopathogens Revista Mexicana de Micología, vol. 40, diciembre, 2014, pp. 27-36 Sociedad Mexicana de Micología Xalapa, México Available in: http://www.redalyc.org/articulo.oa?id=88342644004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Propiedades antagonistas de micromicetos aislados de cenotes de la península de Yucatin contra hongos fitopatógenos Resumen. En la búsqueda de alternativas naturales para el control de enlerrnedades lúngicas, los micromicetos con propiedades antagónicas son considerados una fuente valiosa para detectar modelos novedosos. En el presente esbJdio, un total de 41 micromicetes tropicales se aislaron a partir de restos vegetales sumergidos en cenotes de la Península de Yucatán. Todas las cepas se evaluaron en ensayos antagonistas contra cuatro hongos fitopat6genos (ColJetotrichum g/oeosporioides, Corynespora cassiico/a, Curvularia sp. y Fusarium sp.). los resultados de este ensayo detectaron que 17 aislamientos (41 %) provocaron ~ 50 % de inhibición del crecimiento micelial (Mel ~ 50 %) de al menos uno de los patógenos evaluados. la inhibición más alta lue ocasionada por las cepas Hypocrea lixii OSN - 37 (Mel ~ 61-77 %) YRhizoctonia so/ani OSE-73 (Mel = 5S-64 %) contra todos los objetivos, mientras que PestaJotiopsis mangiferae OH - 02 (51 S9%) causó la inhibición en tres de las cuatro cepas patógenas. -

<I>Cercospora Sojina</I>
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 8-2017 Genetic analysis of field populations of the plant pathogens Cercospora sojina, Corynespora cassiicola and Phytophthora colocasiae Sandesh Kumar Shrestha University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Plant Pathology Commons Recommended Citation Shrestha, Sandesh Kumar, "Genetic analysis of field populations of the plant pathogens Cercospora sojina, Corynespora cassiicola and Phytophthora colocasiae. " PhD diss., University of Tennessee, 2017. https://trace.tennessee.edu/utk_graddiss/4650 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Sandesh Kumar Shrestha entitled "Genetic analysis of field populations of the plant pathogens Cercospora sojina, Corynespora cassiicola and Phytophthora colocasiae." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Entomology and Plant Pathology. Heather M. Young-Kelly, -

GROWING INDOOR PLANTS with Success
GROWING INDOOR PLANTS with Success Table of Contents Introduction............................................................................................................................ 3 Factors Affecting Plant Growth............................................................................................ 3 Light..................................................................................................................................... 3 Temperature........................................................................................................................ 5 Relative Humidity................................................................................................................. 6 Water................................................................................................................................... 7 Water Quantity ................................................................................................................. 7 Water Quality.................................................................................................................... 7 Nutrition ............................................................................................................................... 8 Soil/Growing Medium .......................................................................................................... 9 Growing Mix for Flowering House Plants.......................................................................... 9 Growing Mixes for Foliage Plants.................................................................................... -
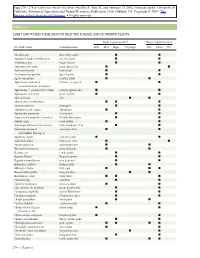
Light and Water Guidelines for Selected Foliage and Flowering Plants
Table 11.1 LIGHT AND WATER GUIDELINES FOR SELECTED FOLIAGE AND FLOWERING PLANTS Light requirements* Water requirements† Scientific name Common name Low Med High Very high Dry Moist Wet Abutilon spp. flowering maple II Acalypha hispida (A.wilkesiana) chenille plant I I Achimenes spp. magic flower II Adiantum cuneatum maidenhair fern II Aechmea fasciata bromeliad II Aeschynanthus pulcher lipstick plant II Agave americana century plant II Aglaonema modestum Chinese evergreen II (A.commutatum,A.simplex) Aglaonema ϫ pseudo-bracteatum golden aglaonema II Aglaonema roebelenii pewter plant II Aloe variegata aloe II Alternanthera bettzickiana II I Ananas comosus pineapple I I Anthurium andreanum anthurium II Aphelandra squarrosa zebra plant II Araucaria heterophylla (A.excelsa) Norfolk Island pine II Ardisia crispa coral ardisia II Asparagus plumosus (A.setaceus) bride’s bouquet fern II Asparagus sprengeri asparagus fern II (A.densiflora Sprenger) Aspidistra elatior cast-iron plant I I Asplenium nidus bird’s nest fern I I Aucuba japonica gold-dust plant I I Beaucarnea recurvata pony tail palm I I Begonia rex rex begonia I I Begonia ‘Rieger’ Rieger begonia I I Begonia semperflorens wax begonia II Beloperone guttata shrimp plant II Billbergia zebrina billbergia III Bougainvillea glabra bougainvillea II Browallia speciosa bush violet II I Caladium spp. caladium II Calathea makoyana peacock plant II Calceolaria herbeahybrida pocketbook plant II Campanula isophylla star-of-Bethlehem II Capsicum annuum Christmas pepper II Carissa grandiflora Natal plum -

Incidence and Biology of Corynespora Cassiicola (Berk
Bangladesh J. Bot. 42(2): 265-272, 2013 (December) INCIDENCE AND BIOLOGY OF CORYNESPORA CASSIICOLA (BERK. & CURT.) WEI. DISEASE OF OKRA IN BANGLADESH FAKHRUDDIN ALI AHMED*, NAZMUL ALAM AND ABUL KHAIR Department of Botany, Jahangirnagar University, Savar, Dhaka-1342, Bangladesh Key words: Incidence, Biology, Corynespora cassiicola, Disease, Okra Abstract Eight commercial okra cultivars were evaluated to determine the magnitude of incidence of Corynespora cassiicola (Berk. & Curt.) Wei. Maximum and significant incidence of C. cassiicola was 14.86 in the cultivar BARI 1. In rest of the cultivars, the occurrence of this fungus was very poor to nil. Corynespora cassiicola appeared to be a minor pathogen for okra. Effects of temperature, pH and culture media on growth and sporulation of the fungus were investigated. Potato dextrose agar medium was found to be the most suitable for optimum growth and sporulation of this fungus at pH 7 and 25ºC. The conidial length significantly varied with the changes of pH. The correlation between pH and conidial length was found negative and highly significant. Introduction Okra (Abelmoschus esculentus (L.) Moench) is a popular and important vegetable crop grown mainly for its tender green fruits in Bangladesh, India and Pakistan. The green fruits are rich in vitamin A and C and minerals like Ca, Mg and Fe. Okra seeds are also good sources of protein and vegetable oil (Yadav and Dhankhar 2001). In Bangladesh, this crop is grown during March to October and suffers from a number of viral and fungal diseases (Ali et al. 2000, Fakir 1976). Fernandes et al. (1990) carried out a preliminary study on health status of okra seeds, collected from different municipalities of Rio de Jenerio state, Brazil and isolated 15 species of pathogenic fungi. -

Plethora of Plants – Collections of the Botanical Garden, Faculty Of
Nat. Croat. Vol. 24(2), 2015 361 NAT. CROAT. VOL. 24 No 2 361–397* ZAGREB December 31, 2015 professional paper / stručni članak – museal collections / muzejske zbirke DOI: 10.302/NC.2015.24.26 PLETHORA OF PLANTS – ColleCtions of the BotaniCal Garden, faCulty of ScienCe, university of ZaGreB (1): temperate Glasshouse exotiCs – HISTORIC OVERVIEW Sanja Kovačić Botanical Garden, department of Biology, faculty of science, university of Zagreb, marulićev trg 9a, HR-10000 Zagreb, Croatia (e-mail: [email protected]) Kovačić, S.: Plethora of plants – collections of the Botanical garden, Faculty of Science, Univer- sity of Zagreb (1): Temperate glasshouse exotics – historic overview. Nat. Croat., Vol. 24, No. 2, 361–397*, 2015, Zagreb due to the forthcoming obligation to thoroughly catalogue and officially register all living and non-living collections in the european union, an inventory revision of the plant collections in Zagreb Botanical Garden of the faculty of science (university of Zagreb, Croatia) has been initiated. the plant lists of the temperate (warm) greenhouse collections since the construction of the first, exhibition Glasshouse (1891), until today (2015) have been studied. synonymy, nomenclature and origin of plant material have been sorted. lists of species grown (or that presumably lived) in the warm greenhouse conditions during the last 120 years have been constructed to show that throughout that period at least 1000 plant taxa from 380 genera and 90 families inhabited the temperate collections of the Garden. today, that collection holds 320 exotic taxa from 146 genera and 56 families. Key words: Zagreb Botanical Garden, warm greenhouse conditions, historic plant collections, tem- perate glasshouse collection Kovačić, S.: Obilje bilja – zbirke Botaničkoga vrta Prirodoslovno-matematičkog fakulteta Sve- učilišta u Zagrebu (1): Uresnice toplog staklenika – povijesni pregled. -

Target Spot of Tomato
DISEASE MANAGEMENT: Target Spot of Tomato Corynespora cassiicola SIGNS & SYMPTOMS: • On leaves, the disease first appears as small, necrotic lesions with light-brown centers and dark margins. • Symptoms often begin deep within the tomato canopy. • On fruit, lesions first appear as brown, slightly sunken flecks. As lesions develop, large, pitted areas appear on fruit. DISEASE CYCLE & EPIDEMIOLOGY: • Target spot is favored by moderate temperatures (70-80°F). • Sandblasting of fruit predisposes tomato fruit to infection. • C. cassiicola has a wide host range, which includes cucumber, papaya and a number of ornamentals. Several common weed species also serve as alternate hosts. FIELD SIGNATURE: • Always inspect the interior of the tomato canopy for the “melting out” effect often seen with target spot. This refers to the loss of foliage in the inside of the canopy which operates to thin out the interior foliage due to premature defoliation. • Pitted fruit may appear to have been damaged by hail or other abiotic stress. PHOTOS: Figure. 1. Typical foliar symptoms of target spot. Photograph by: Ken Pernezny. Figure 2. Severe pitting of tomato fruit due to target spot. Photograph by: Ken Pernezny. Figure 3. Small pits in green fruit associated with target spot. Photograph by: Ken Pernezny. Prepared by: Dr. Ken Pernezny 119 DISEASE MANAGEMENT: Target Spot of Tomato CULTURAL CONTROLS: CHEMICAL CONTROL: • Destroy crop residues promptly. • Chlorothalonil and mancozeb (maneb) provide fairly good control of target spot when applied on a • Avoid overfertilization, especially with nitrogen, as preventative basis. this leads to a lush growth habit, with more likelihood of significant “melting out”. • New chemistries, including the strobilurins and related compounds (e.g., azoxystrobin and famoxadone + • Be certain that fields are scouted thoroughly and cymoxanil), have given excellent control of target that target spot is not misdiagnosed as bacterial spot, spot in University research trials. -

Fungicide Sensitivity of Corynespora Cassiicola and Assessment Of
FUNGICIDE SENSITIVITY OF CORYNESPORA CASSIICOLA AND ASSESSMENT OF MANAGEMENT OF TARGET SPOT OF COTTON IN GEORGIA by: MA. KATRINA SHIELA E. LAUREL (Under the Direction of Robert C. Kemerait, Jr.) ABSTRACT Target spot, caused by Corynespora cassiicola, is a serious foliar disease of cotton in the southeastern United States. Baseline (current) isolates of C. cassiicola were tested for sensitivity to metconazole (DMI), fluxapyroxad (SDHI) and pyraclostrobin (QoI). Further work compared fungicide sensitivity of C. cassiicola isolates from cotton to isolates from other hosts. Field experiments were conducted to establish a relationship between fungicide sensitivity in laboratory experiments and fungicide efficacy in managing target spot on cotton. Based on the sensitivity distribution, all isolates tested were considered sensitive to fungicides. However, these sensitivities varied among isolates offering an early indication that resistance can happen in the future. Additionally, all fungicides reduced disease severity and premature defoliation; however, Priaxor (pyraclostrobin + fluxapyroxad [QoI + SDHI]) proved to be most effective. Results from this study can help optimize fungicide sensitivity monitoring practices in an effort to improve fungicide use patterns for optimum disease management. INDEX WORDS: Corynespora cassiicola, Cotton, DMIs, Fluxapyroxad, Fungicide Resistance, Metconazole, Pyraclostrobin, QoIs, SDHIs, Target spot FUNGICIDE SENSITIVITY OF CORYNESPORA CASSIICOLA AND ASSESSMENT OF MANAGEMENT OF TARGET SPOT OF COTTON IN GEORGIA by: MA. KATRINA SHIELA E. LAUREL B.S., Southern Luzon State University, Lucban, Philippines, 2010 A Thesis Submitted to the Graduate Faculty of The University of Georgia in Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE ATHENS, GEORGIA 2018 ©2018 Ma. Katrina Shiela E. Laurel All Rights Reserved FUNGICIDE SENSITIVITY OF CORYNESPORA CASSIICOLA AND ASSESSMENT OF MANAGEMENT OF TARGET SPOT OF COTTON IN GEORGIA by: MA. -
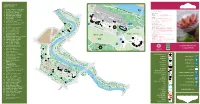
The-Commonwealth-Collection.Pdf
Round Border COMMONWEALTH Fern COLLECTION Garden Gate River W 1. Protea cyanoides - South Africa alk 2. Strelitzia reginae - South Africa GENERAL INFORMATION 3. Acacia karroo - Zambia Long Pit Glasshouse OPENING HOURS 4. Banksia ericifolia - Australia 32 GROUNDS 7 am to dusk (all year) 5. Wollemia nobilis - Australia 33 31 GLASSHOUSES 10 am - 6 pm (4.15 pm in winter) 30 6. Leptospermum scoparium - CHARGES 29 stairs New Zealand Hopkirk Glasgow City Council maintains a policy of free entry Building 7. Cyathea arborea - Jamaica 28 THE ENQUIRIES OFFICE: is situated behind the main range 27 25 15 and further information may be obtained there. 11 Enquiries 8. Ficus carica - Malta 26 24 23 20 21 22 10 19 Office PHONE: 0141 276 1614 9. Phormium tenax - New Zealand 17 Main Range FAX: 0141 276 1615 18 Glasshouses Curator’s 10. Angraecum podochiloides - 16 14 EMAIL: [email protected] House WEB: glasgowbotanicgardens.com or 13 Private Cameroon 12 www.glasgow.gov.uk/parks 11. Ansellia africana - Zambia 9 GROUPS VISITS: are especially welcome and a guide may be 12. Bowiea volubilis - Kenya Botanic Gardens available if arranged in advance with the Gardens’ Office. 13. Cycas seemannii - Fiji Tea Room DOGS: are allowed in the grounds, but should be kept on a 14. Ficus benjamina - India short leash. Dogs are not permitted in the glasshouses with the Peter exception of guide dogs. 15. Heliconia psittacorum - Walker TRANSPORT: by bus from the City Centre nos. 6, 6a, 6b, Trinidad & Tobago Memorial QUEEN 8, 8a, 10a, and 19. 16. Elettaria cardamomum - Sri Lanka MARGARET By Underground - to Hillhead Station 17.