Baseline Sensitivities of Corynespora Cassiicola to Thiophanate-Methyl, Iprodione and Fludioxonil
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Estrategias De Manejo De La Roña Cladosporium Cladosporioides (FRESEN) G.A
Estrategias de Manejo de la Roña Cladosporium cladosporioides (FRESEN) G.A. de VRIES de la Gulupa Passiflora edulis f. edulis Sims. Carlos Fernando Castillo Londoño Universidad Nacional de Colombia Facultad de Ciencias Agropecuarias Maestría en Ciencias Agrarias Palmira, 2014 Estrategias de Manejo de la Roña Cladosporium cladosporioides (FRESEN) G.A. de VRIES de la Gulupa Passiflora edulis f. edulis Sims. Carlos Fernando Castillo Londoño Trabajo de investigación presentado como requisito parcial para optar al título de: Magister en Ciencias Agrarias con énfasis en Fitopatología Director: Ph.D. Carlos German Muñoz Perea Codirector: Ph.D. Elizabeth Álvarez Codirector: Ph.D. Kriss Wichechkus Universidad Nacional de Colombia Facultad de Ciencias Agropecuarias Maestría en Ciencias Agrarias Palmira. 2014 A Dios por darme la inmensa voluntad y capacidad de entrega para la hacer las cosas con amor y dedicación. A mi madre Carmen y a Geovanny por su gran confianza, apoyo, consejos y su constante exigencia para formar la persona que soy. AGRADECIMIENTOS A la Universidad Nacional de Colombia sede Palmira por las oportunidades y facilidades brindadas en el transcurso de mi carrera profesional. A la Universidad Jorge Tadeo Lozano y al Centro Internacional de Agricultura Tropical (CIAT) por brindarme la oportunidad de trabajar en el proyecto. A mi director Carlos German Muñoz, y codirectores Elizabeth Álvarez y Kriss Wichechkus, por sus valiosos aportes y orientación en esta investigación. A la Dra. Martha Cárdenas directora del Centro de Transformación Genética (Riverside), por su valiosa colaboración en la revisión del documento. A mis profesores, a quienes les debo gran parte de mis conocimientos, gracias por prepararnos para un futuro competitivo no solo como los mejores profesionales sino también como mejores personas. -

Phaeoseptaceae, Pleosporales) from China
Mycosphere 10(1): 757–775 (2019) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/10/1/17 Morphological and phylogenetic studies of Pleopunctum gen. nov. (Phaeoseptaceae, Pleosporales) from China Liu NG1,2,3,4,5, Hyde KD4,5, Bhat DJ6, Jumpathong J3 and Liu JK1*,2 1 School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 611731, P.R. China 2 Guizhou Key Laboratory of Agricultural Biotechnology, Guizhou Academy of Agricultural Sciences, Guiyang 550006, P.R. China 3 Faculty of Agriculture, Natural Resources and Environment, Naresuan University, Phitsanulok 65000, Thailand 4 Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand 5 Mushroom Research Foundation, Chiang Rai 57100, Thailand 6 No. 128/1-J, Azad Housing Society, Curca, P.O., Goa Velha 403108, India Liu NG, Hyde KD, Bhat DJ, Jumpathong J, Liu JK 2019 – Morphological and phylogenetic studies of Pleopunctum gen. nov. (Phaeoseptaceae, Pleosporales) from China. Mycosphere 10(1), 757–775, Doi 10.5943/mycosphere/10/1/17 Abstract A new hyphomycete genus, Pleopunctum, is introduced to accommodate two new species, P. ellipsoideum sp. nov. (type species) and P. pseudoellipsoideum sp. nov., collected from decaying wood in Guizhou Province, China. The genus is characterized by macronematous, mononematous conidiophores, monoblastic conidiogenous cells and muriform, oval to ellipsoidal conidia often with a hyaline, elliptical to globose basal cell. Phylogenetic analyses of combined LSU, SSU, ITS and TEF1α sequence data of 55 taxa were carried out to infer their phylogenetic relationships. The new taxa formed a well-supported subclade in the family Phaeoseptaceae and basal to Lignosphaeria and Thyridaria macrostomoides. -

Ekspedisi Saintifik Biodiversiti Hutan Paya Gambut Selangor Utara 28 November 2013 Hotel Quality, Shah Alam SELANGOR D
Prosiding Ekspedisi Saintifik Biodiversiti Hutan Paya Gambut Selangor Utara 28 November 2013 Hotel Quality, Shah Alam SELANGOR D. E. Seminar Ekspedisi Saintifik Biodiversiti Hutan Paya Gambut Selangor Utara 2013 Dianjurkan oleh Jabatan Perhutanan Semenanjung Malaysia Jabatan Perhutanan Negeri Selangor Malaysian Nature Society Ditaja oleh ASEAN Peatland Forest Programme (APFP) Dengan Kerjasama Kementerian Sumber Asli and Alam Sekitar (NRE) Jabatan Perlindungan Hidupan Liar dan Taman Negara (PERHILITAN) Semenanjung Malaysia PROSIDING 1 SEMINAR EKSPEDISI SAINTIFIK BIODIVERSITI HUTAN PAYA GAMBUT SELANGOR UTARA 2013 ISI KANDUNGAN PENGENALAN North Selangor Peat Swamp Forest .................................................................................................. 2 North Selangor Peat Swamp Forest Scientific Biodiversity Expedition 2013...................................... 3 ATURCARA SEMINAR ........................................................................................................................... 5 KERTAS PERBENTANGAN The Socio-Economic Survey on Importance of Peat Swamp Forest Ecosystem to Local Communities Adjacent to Raja Musa Forest Reserve ........................................................................................ 9 Assessment of North Selangor Peat Swamp Forest for Forest Tourism ........................................... 34 Developing a Preliminary Checklist of Birds at NSPSF ..................................................................... 41 The Southern Pied Hornbill of Sungai Panjang, Sabak -

Evidence That the Biocontrol Agent Bacillus Cereus Synthesizes Protein That Can Elicit Increased Resistance of Tomato Leaves to Corynespora Cassiicola
Tropical Plant Pathology, vol. 35, 1, 011-015 (2010) Copyright by the Brazilian Phytopathological Society. Printed in Brazil www.sbfito.com.br RESEARCH ARTICLE / ARTIGO Evidence that the biocontrol agent Bacillus cereus synthesizes protein that can elicit increased resistance of tomato leaves to Corynespora cassiicola Reginaldo S. Romeiro1, Roberto Lanna Filho1, Dirceu Macagnan2, Flávio A.O. Garcia1 & Harllen S.A. Silva3 1Departamento de Fitopatologia, Universidade Federal de Viçosa, 36570-000 Viçosa, MG, Brazil; 2Centro Federal de Educação Tecnológica de Rio Verde, 75901-970, Rio Verde, GO, Brazil; 3Embrapa Mandioca e Fruticultura, 44380-000, Cruz das Almas, BA, Brazil Author for correspondence: Dirceu Macagnan, e-mail: [email protected] ABSTRACT Isolate UFV-101 of Bacillus cereus was selected in previous studies for promoting growth inducing resistance in plants. In a previous study, supernatant from cultures of the microorganism in a liquid medium was found to induce resistance in tomato foliage against the pathogens Pseudomonas syringae pv. tomato, Xanthomonas vesicatoria, Alternaria solani and Corynespora cassiicola. In the present work the microorganism was grown in a minimal medium for 48 h and the cells precipitated for centrifugation. The supernatant was concentrated by lyophilization, dialyzed in a 12 kDa cut-off point membrane and fractioned in column containing Sephadex G25 balanced in PBS buffer. The fractions corresponding to a protein peak were applied to tomato seedlings. After four days leaflets were collected and inoculated with the pathogen. C. cassiicola. The numbers of lesions produced by the pathogen on leaflets exposed to the bacterial supernatant were similar to those exposed to acibenzolar-S-methyl but fewer than in those treated with water. -

Aeschynanthus Pulcher Lipstick Plant LIPSTICK PLANT
The Gardener’s Resource 435 W. Glenside Ave. Since 1943 Glenside, PA 19038 215-887-7500 Aeschynanthus pulcher Lipstick Plant LIPSTICK PLANT Lipstick plants are easy indoor flowering houseplants that when given the right amount of light and water, they produce numerous red or orange small tubular flowers that resemble a tube of lipstick. Not only are the flowers colorful, the leaves can be light green, dark green or green and maroon. Light: The Lipstick vine will not bloom without adequate light. They require very bright, indirect light. Avoid placing this plant in full shade or full sun. Direct sun will burn the leaves. The plant needs bright light for a portion of the day, but not all day long. Water: If you allow the top 25% of the soil to dry out before watering, this plant will flower more frequently and more abundant. If the leaves appear soft and shriveled, give it more water. These plants will also lose green leaves TIPS: when over-watered. • Lipstick plants like warm temperatures between 75-85°F. : Fertilizer every other week in the Fertilizing • Prefers high humidity, but will do well in Spring and Summer and only monthly in the basic household humidity too. Fall and Winter with a houseplant food high in • Trim the long vines to prevent the plant phosphorous. Always dilute the fertilizer to ½ from becoming thin and straggly. the recommended strength. • Lipstick Plants are Non-Poisonous. • Keep a lipstick plant in a small pot will help it produce more flowers. When put into a large container, instead of producing flowers it grows more leaves. -

Pseudodidymellaceae Fam. Nov.: Phylogenetic Affiliations Of
available online at www.studiesinmycology.org STUDIES IN MYCOLOGY 87: 187–206 (2017). Pseudodidymellaceae fam. nov.: Phylogenetic affiliations of mycopappus-like genera in Dothideomycetes A. Hashimoto1,2, M. Matsumura1,3, K. Hirayama4, R. Fujimoto1, and K. Tanaka1,3* 1Faculty of Agriculture and Life Sciences, Hirosaki University, 3 Bunkyo-cho, Hirosaki, Aomori, 036-8561, Japan; 2Research Fellow of the Japan Society for the Promotion of Science, 5-3-1 Kojimachi, Chiyoda-ku, Tokyo, 102-0083, Japan; 3The United Graduate School of Agricultural Sciences, Iwate University, 18–8 Ueda 3 chome, Morioka, 020-8550, Japan; 4Apple Experiment Station, Aomori Prefectural Agriculture and Forestry Research Centre, 24 Fukutami, Botandaira, Kuroishi, Aomori, 036-0332, Japan *Correspondence: K. Tanaka, [email protected] Abstract: The familial placement of four genera, Mycodidymella, Petrakia, Pseudodidymella, and Xenostigmina, was taxonomically revised based on morphological observations and phylogenetic analyses of nuclear rDNA SSU, LSU, tef1, and rpb2 sequences. ITS sequences were also provided as barcode markers. A total of 130 sequences were newly obtained from 28 isolates which are phylogenetically related to Melanommataceae (Pleosporales, Dothideomycetes) and its relatives. Phylo- genetic analyses and morphological observation of sexual and asexual morphs led to the conclusion that Melanommataceae should be restricted to its type genus Melanomma, which is characterised by ascomata composed of a well-developed, carbonaceous peridium, and an aposphaeria-like coelomycetous asexual morph. Although Mycodidymella, Petrakia, Pseudodidymella, and Xenostigmina are phylogenetically related to Melanommataceae, these genera are characterised by epi- phyllous, lenticular ascomata with well-developed basal stroma in their sexual morphs, and mycopappus-like propagules in their asexual morphs, which are clearly different from those of Melanomma. -

Redalyc.Antagonistic Propierties of Micromycetes Isolated From
Revista Mexicana de Micología ISSN: 0187-3180 [email protected] Sociedad Mexicana de Micología México Moreno-Pérez, Pablo; Gamboa-Angulo, Marcela; Heredia, Gabriela; Canto-Canché, Blondy; Rosado-Vallado, Miguel; Medina-Baizabal, Irma L.; Tapia-Tusell, Raúl Antagonistic propierties of micromycetes isolated from sinkholes of the Yucatán Península against fungal phytopathogens Revista Mexicana de Micología, vol. 40, diciembre, 2014, pp. 27-36 Sociedad Mexicana de Micología Xalapa, México Available in: http://www.redalyc.org/articulo.oa?id=88342644004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Propiedades antagonistas de micromicetos aislados de cenotes de la península de Yucatin contra hongos fitopatógenos Resumen. En la búsqueda de alternativas naturales para el control de enlerrnedades lúngicas, los micromicetos con propiedades antagónicas son considerados una fuente valiosa para detectar modelos novedosos. En el presente esbJdio, un total de 41 micromicetes tropicales se aislaron a partir de restos vegetales sumergidos en cenotes de la Península de Yucatán. Todas las cepas se evaluaron en ensayos antagonistas contra cuatro hongos fitopat6genos (ColJetotrichum g/oeosporioides, Corynespora cassiico/a, Curvularia sp. y Fusarium sp.). los resultados de este ensayo detectaron que 17 aislamientos (41 %) provocaron ~ 50 % de inhibición del crecimiento micelial (Mel ~ 50 %) de al menos uno de los patógenos evaluados. la inhibición más alta lue ocasionada por las cepas Hypocrea lixii OSN - 37 (Mel ~ 61-77 %) YRhizoctonia so/ani OSE-73 (Mel = 5S-64 %) contra todos los objetivos, mientras que PestaJotiopsis mangiferae OH - 02 (51 S9%) causó la inhibición en tres de las cuatro cepas patógenas. -

Genome-Wide Analysis of Corynespora Cassiicola Leaf Fall Disease Putative Effectors
Lawrence Berkeley National Laboratory Recent Work Title Genome-Wide Analysis of Corynespora cassiicola Leaf Fall Disease Putative Effectors. Permalink https://escholarship.org/uc/item/76h0p216 Journal Frontiers in microbiology, 9(MAR) ISSN 1664-302X Authors Lopez, David Ribeiro, Sébastien Label, Philippe et al. Publication Date 2018 DOI 10.3389/fmicb.2018.00276 Peer reviewed eScholarship.org Powered by the California Digital Library University of California ORIGINAL RESEARCH published: 02 March 2018 doi: 10.3389/fmicb.2018.00276 Genome-Wide Analysis of Corynespora cassiicola Leaf Fall Disease Putative Effectors David Lopez 1, Sébastien Ribeiro 1,2,3, Philippe Label 1, Boris Fumanal 1, Jean-Stéphane Venisse 1, Annegret Kohler 4, Ricardo R. de Oliveira 5, Kurt Labutti 6, Anna Lipzen 6, Kathleen Lail 6, Diane Bauer 6, Robin A. Ohm 6,7, Kerrie W. Barry 6, Joseph Spatafora 8, Igor V. Grigoriev 6,9, Francis M. Martin 4 and Valérie Pujade-Renaud 1,2,3* 1 Université Clermont Auvergne, Institut National de la Recherche Agronomique, UMR PIAF, Clermont-Ferrand, France, 2 CIRAD, UMR AGAP, Clermont-Ferrand, France, 3 AGAP, Université Montpellier, CIRAD, Institut National de la Recherche Agronomique, Montpellier SupAgro, Montpellier, France, 4 Institut National de la Recherche Agronomique, UMR INRA-Université de Lorraine “Interaction Arbres/Microorganismes,” Champenoux, France, 5 Departemento de Agronomia, Universidade Estadual de Maringá, Maringá, Brazil, 6 United States Department of Energy Joint Genome Institute, Walnut Creek, CA, United States, 7 Department of Microbiology, Utrecht University, Utrecht, Netherlands, 8 Department of Botany and Plant Pathology, Oregon State University, Corvallis, OR, United States, 9 Department of Plant and Microbial Biology, University of California, Berkeley, Berkeley, CA, United States Corynespora cassiicola is an Ascomycetes fungus with a broad host range and diverse life styles. -

<I>Cercospora Sojina</I>
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 8-2017 Genetic analysis of field populations of the plant pathogens Cercospora sojina, Corynespora cassiicola and Phytophthora colocasiae Sandesh Kumar Shrestha University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Plant Pathology Commons Recommended Citation Shrestha, Sandesh Kumar, "Genetic analysis of field populations of the plant pathogens Cercospora sojina, Corynespora cassiicola and Phytophthora colocasiae. " PhD diss., University of Tennessee, 2017. https://trace.tennessee.edu/utk_graddiss/4650 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Sandesh Kumar Shrestha entitled "Genetic analysis of field populations of the plant pathogens Cercospora sojina, Corynespora cassiicola and Phytophthora colocasiae." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Entomology and Plant Pathology. Heather M. Young-Kelly, -

GROWING INDOOR PLANTS with Success
GROWING INDOOR PLANTS with Success Table of Contents Introduction............................................................................................................................ 3 Factors Affecting Plant Growth............................................................................................ 3 Light..................................................................................................................................... 3 Temperature........................................................................................................................ 5 Relative Humidity................................................................................................................. 6 Water................................................................................................................................... 7 Water Quantity ................................................................................................................. 7 Water Quality.................................................................................................................... 7 Nutrition ............................................................................................................................... 8 Soil/Growing Medium .......................................................................................................... 9 Growing Mix for Flowering House Plants.......................................................................... 9 Growing Mixes for Foliage Plants.................................................................................... -
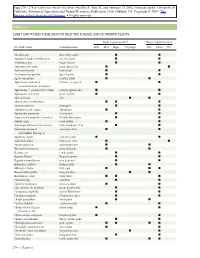
Light and Water Guidelines for Selected Foliage and Flowering Plants
Table 11.1 LIGHT AND WATER GUIDELINES FOR SELECTED FOLIAGE AND FLOWERING PLANTS Light requirements* Water requirements† Scientific name Common name Low Med High Very high Dry Moist Wet Abutilon spp. flowering maple II Acalypha hispida (A.wilkesiana) chenille plant I I Achimenes spp. magic flower II Adiantum cuneatum maidenhair fern II Aechmea fasciata bromeliad II Aeschynanthus pulcher lipstick plant II Agave americana century plant II Aglaonema modestum Chinese evergreen II (A.commutatum,A.simplex) Aglaonema ϫ pseudo-bracteatum golden aglaonema II Aglaonema roebelenii pewter plant II Aloe variegata aloe II Alternanthera bettzickiana II I Ananas comosus pineapple I I Anthurium andreanum anthurium II Aphelandra squarrosa zebra plant II Araucaria heterophylla (A.excelsa) Norfolk Island pine II Ardisia crispa coral ardisia II Asparagus plumosus (A.setaceus) bride’s bouquet fern II Asparagus sprengeri asparagus fern II (A.densiflora Sprenger) Aspidistra elatior cast-iron plant I I Asplenium nidus bird’s nest fern I I Aucuba japonica gold-dust plant I I Beaucarnea recurvata pony tail palm I I Begonia rex rex begonia I I Begonia ‘Rieger’ Rieger begonia I I Begonia semperflorens wax begonia II Beloperone guttata shrimp plant II Billbergia zebrina billbergia III Bougainvillea glabra bougainvillea II Browallia speciosa bush violet II I Caladium spp. caladium II Calathea makoyana peacock plant II Calceolaria herbeahybrida pocketbook plant II Campanula isophylla star-of-Bethlehem II Capsicum annuum Christmas pepper II Carissa grandiflora Natal plum -

1 Check-List of Cladosporium Names Frank M. DUGAN, Konstanze
Check-list of Cladosporium names Frank M. DUGAN , Konstanze SCHUBERT & Uwe BRAUN Abstract: DUGAN , F.M., SCHUBERT , K. & BRAUN ; U. (2004): Check-list of Cladosporium names. Schlechtendalia 11 : 1–119. Names of species and subspecific taxa referred to the hyphomycetous genus Cladosporium are listed. Citations for original descriptions, types, synonyms, teleomorphs (if known), references of important redescriptions in literature, illustrations as well as notes are given. This list contains data of 772 taxa, i.e., valid, invalid and illegitime species, varieties and formae as well as herbarium names. Zusammenfassung: DUGAN , F.M., SCHUBERT , K. & BRAUN ; U. (2004): Checkliste der Cladosporium -Namen. Schlechtendalia 11 : 1–119. Namen von Arten und subspezifischen Taxa der Hyphomycetengattung Cladosporium werden aufgelistet. Bibliographische Angaben zur Erstbeschreibung, Typusangaben, Synonyme, die Teleomorphe (falls bekannt), wichtige Literaturhinweise und Abbildungen sowie Anmerkungen werden angegeben. Die vorliegende Liste enthält Namen von 772 Taxa, d. h. gültige, ungültige und illegitime Arten, Varietäten, Formen und auch Herbarnamen. Introduction: Cladosporium Link (LINK 1816) is one of the largest genera of hyphomycetes, comprising more than 772 names, but also one of the most heterogeneous ones, which is not very surprising since all early circumscriptions and delimitations from similar genera were rather vague and imprecise (FRIES 1832, 1849; SACCARDO 1886; LINDAU 1907, etc.). All kinds of superficially similar cladosporioid fungi, i.e., amero- to phragmosporous dematiaceous hyphomycetes with conidia formed in acropetal chains, were assigned to Cladosporium s. lat., ranging from saprobes to plant pathogens as well as human-pathogenic taxa. DE VRIES (1952) and ELLIS (1971, 1976) maintained broad concepts of Cladosporium . ARX (1983), MORGAN - JONES & JACOBSEN (1988), MCKEMY & MORGAN -JONES (1990), MORGAN -JONES & MCKEMY (1990) and DAVID (1997) discussed the heterogeneity of Cladosporium and contributed towards a more natural circumscription of this genus.