Reanalyzing the 1900–1920 Sleeping Sickness Epidemic in Uganda E.M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Whether a Rwandan Born in Uganda Has Citizenship Rights
Home > Research > Responses to Information Requests RESPONSES TO INFORMATION REQUESTS (RIRs) New Search | About RIRs | Help 22 January 2008 ZZZ102691.E Uganda/Rwanda: Whether a Rwandan born in Uganda has citizenship rights; conditions under which individuals whose ancestors are not Ugandan can obtain Ugandan citizenship; whether citizens of Uganda have rights to free university education; whether international students can attend university in Uganda Research Directorate, Immigration and Refugee Board of Canada, Ottawa Citizenship rights A Rwandan born in Uganda is not automatically granted Ugandan citizenship (Uganda 18 Jan. 2008; ibid. 1995, Chapter 3, Sec. 10; US Mar. 2001, 205). Under Chapter 3, Section 10 of the Constitution of the Republic of Uganda, 1995, persons granted Ugandan citizenship by birth include: (a) every person born in Uganda one of whose parents or grandparents is or was a member of any of the indigenous communities existing and residing within the borders of Uganda at the first day of February, 1926, and set out in the Third Schedule to this Constitution; and (b) every person born in or outside Uganda one of whose parents or grandparents was at the time of birth of that person a citizen of Uganda by birth. The constitution also outlines several conditions under which an individual whose ancestors are not Ugandan can obtain Ugandan citizenship (Uganda 1995, Chapter 3, Sec. 11-12). For example, abandoned children of no more than five years of age, whose parents are unknown, are considered citizens of the country (ibid., Chapter 3, Sec. 11). An individual under the age of 18 years who is adopted by a Ugandan citizen may also be granted citizenship (ibid.). -

The Populat Kenya
£ 4 World Population Year THE POPULAT KENYA - UGANDA - TANZANIA CI.CR.E.D. Senes THE POPULATION OF KENYA- UGANDA - TANZANIA SIMEON OMINDE Professor of Geography and Head of Department, University of Nairobi 1974 World Population Year C.I.C.R.E.D Series This study was initiated and financed by C.I.C.R.E.D. (Committee for International Coordination of National Research in Demography) to coincide with 1974 World Population Year. © Simeon Ominde © C.I.C.R.E.D. First published 1975 Printed in Kenya by Kenya Litho Ltd., P.O. Box 40775, Changamwe Road, Nairobi. CONTENTS Page PREFACE ¡v Chapter 1 The Area and Estimates of Population Growth 1 Chapter 2 Components of Population Growth 11 Chapter 3 Migration 40 Chapter 4 Population Composition 59 Chapter 5 Population Distribution 73 Chapter 6 Urbanization 88 Chapter 7 Labour Force 97 Chapter 8 Population Projection 105 Chapter 9 Population Growth and Socio-Economic Development 115 Conclusion 123 PREFACE This monograph presents the population situation in the area covered by Tanzania, Uganda and Kenya. The material has been prepared at the request of CICRED, as part of its contribution to the objectives of the World Population Year, 1974. In common with other developing countries of Africa, the East African countries are becoming acutely aware of the importance of rapid population growth and its significance to the attainment of development objectives. It has become increasingly clear that with the current rates of growth and the limited resources, the burden of socio-economic development programmes has become more serious. The search for alternative strategies to development must therefore focus attention on the impact of accelerating growth rate which leads to retardation of the rate of economic and social development. -

Double Taxation Treaties in Uganda Impact and Policy Implications
Double Taxation Treaties in Uganda Impact and Policy Implications July 2014 Double Taxation Treaties in Uganda Impact and Policy Implications Double Taxation Treaties in Uganda Impact and Policy Implications July 2014 3 Double Taxation Treaties in Uganda Impact and Policy Implications Table of Contents List of Abbreviations 5 Executive Summary 7 Introduction 9 Comparing the UN and OECD Double Taxation Conventions 11 Double Taxation Treaties in Uganda 13 Analyses of Selected Clauses of the Two Treaties 15 Treaty Abuses can Lead to Major Tax Losses 20 Successes with Renegotiations and Cancellations 21 Conclusion 23 Policy Recommendations for Renegotiating Positions 25 References 28 List of boxes and tables Box 1: The cost of treaty shopping to Zambia 10 Box 2: Indian round tripping through Mauritius 10 Table 1: Permanent Establishment Continuum 17 Table 2: Withholding Tax Rates 18 Table 3: Withholding Tax Rate Continuum 18 Table 4: Capital Gains Continuum 19 4 Double Taxation Treaties in Uganda Impact and Policy Implications List of abbreviations CGT Capital Gains Tax DTA(s) Double Taxation Agreement(s) DTT(s) Double Taxation Treaty (Treaties) EAC East African Community FDI Foreign Direct Investment ITA Income Tax Act, 1997 LDC Least Developed Country (Countries) MNC Multinational Companies MDG Millennium Development Goals MU Mauritius NL The Netherlands OECD Organisation for Economic Cooperation and Development PE Permanent Establishment RSA Republic of South Africa UAE United Arab Emirates UIA Uganda Investment Authority URA Uganda Revenue Authority 5 Double Taxation Treaties in Uganda Impact and Policy Implications Acknowledgments This paper was produced jointly by SEATINI-Uganda and ActionAid International Uganda. We extend our appreciation to the following for their contributions towards the production of this report: Ms Jalia Kangave, Ms Ruth Kelly, Mr Moses Kawumi, Mr Anders Reimers Larsen, Ms Sarah Muyonga, Ms Jane Nalunga, Ms Nelly Busingye Mugisha, Ms Regina Navuga and members of the Tax Justice Taskforce. -

The Charcoal Grey Market in Kenya, Uganda and South Sudan (2021)
COMMODITY REPORT BLACK GOLD The charcoal grey market in Kenya, Uganda and South Sudan SIMONE HAYSOM I MICHAEL McLAGGAN JULIUS KAKA I LUCY MODI I KEN OPALA MARCH 2021 BLACK GOLD The charcoal grey market in Kenya, Uganda and South Sudan ww Simone Haysom I Michael McLaggan Julius Kaka I Lucy Modi I Ken Opala March 2021 ACKNOWLEDGEMENTS The authors would like to thank everyone who gave their time to be interviewed for this study. They would like to extend particular thanks to Dr Catherine Nabukalu, at the University of Pennsylvania, and Bryan Adkins, at UNEP, for playing an invaluable role in correcting our misperceptions and deepening our analysis. We would also like to thank Nhial Tiitmamer, at the Sudd Institute, for providing us with additional interviews and information from South Sudan at short notice. Finally, we thank Alex Goodwin for excel- lent editing. Interviews were conducted in South Sudan, Uganda and Kenya between February 2020 and November 2020. ABOUT THE AUTHORS Simone Haysom is a senior analyst at the Global Initiative Against Transnational Organized Crime (GI-TOC), with expertise in urban development, corruption and organized crime, and over a decade of experience conducting qualitative fieldwork in challenging environments. She is currently an associate of the Oceanic Humanities for the Global South research project based at the University of the Witwatersrand in Johannesburg. Ken Opala is the GI-TOC analyst for Kenya. He previously worked at Nation Media Group as deputy investigative editor and as editor-in-chief at the Nairobi Law Monthly. He has won several journalistic awards in his career. -

Country Reports
COUNTRY REPORTS DISPLACED & DISCONNECTED UNHCR CONNECTIVITY FOR REFUGEES Innovation Service COUNTRY REPORTS DISPLACED AND DISCONNECTED Made possible thanks to the generous support of: In partnership with: The GSMA represents the interests of mobile operators worldwide, uniting more than 750 operators with over 350 companies in the broader mobile ecosystem, including handset and device makers, software companies, equipment providers and internet companies, as well as organisations in adjacent industry sectors. The GSMA also produces the industry-leading MWC events held annually in Barcelona, Los Angeles and Shanghai, as well as the Mobile 360 Series of regional conferences. For more information, please visit the GSMA corporate website at www.gsma.com Follow the GSMA on Twitter: @GSMA TABLE OF CONTENTS INTRODUCTION INTRODUCTION 1 These country reports provide information on the legal situation for displaced populations, namely AFGHANISTAN 2 asylum seekers, refugees, and returnees, where relevant, regarding access to mobile services, in BANGLADESH 6 each country covered. Each report contains information on: BRAZIL 10 BURUNDI 14 • Registration and Identification of Displaced Persons CAMEROON 18 • SIM Registration CENTRAL AFRICAN REPUBLIC 22 • Know Your Customer Rules • Mobile Money CHAD 26 • Data Protection DEMOCRATIC REPUBLIC OF CONGO 30 ETHIOPIA 34 Information sources cover freely published information from the relevant regulators or ministries, JORDAN 38 academic papers and other internet sources. Specific information on the practical situation in KENYA 42 country has been provided by UNHCR local staff. LEBANON 46 MAURITANIA 50 The content of this report, including information and links, is provided free of charge and is NIGER 54 intended to be helpful to the widest range of people and organizations. -

Ghana South Africa Uganda
Connecting the Globe: The Africa Initiative Appendix 4 THE TELECOMMUNICATIONS SECTORS IN GHANA, SOUTH AFRICA AND UGANDA Ghana Ghana sold an equity stake of 30% and management control of the state’s sole service provider and licensed a second national operator in order to facilitate private-sector-led expansion of telecommunications infrastructure and services nationwide. The National Communications Authority Act of 1996, which went into effect in 1997, created Ghana’s regulator, the Ghana National Communications Authority (GNCA). The regulator reports to the Ministry of Transport and Communications. GNCA is financed by license fees, spectrum fees and funds from parliament. The responsibilities of GNCA include licensing, number planning, tariff approval and establishing licensing fees together with the sector ministry, interconnection rates together with the sector ministry and the operator, technical standards, frequency allocation, type approval, and service quality monitoring. Ghana has full competition in cellular services, and duopolies in local, domestic long distance and international services. Ghana is a WTO member that has signed the Basic Telecom Services Agreement. South Africa The separation of Posts and Telecom occurred in 1991 with the formation of Telkom. The Telecommunications Act, Act 103 of 1996 created an independent regulator, the South African Telecommunications Regulatory Authority (SATRA). The policy maker is the Ministry for Posts, Telecommunications, and Broadcasting and the head of SATRA reports to Ministry of Communications. SATRA is financed by parliamentary appropriations and its responsibilities include licensing, number planning, tariff approval, technical standards, frequency allocation, type approval, monitoring service quality and establishing license fees. South Africa has a monopoly in basic telecommunications services, a duopoly in cellular services. -

Study Abroad Uganda-Rwanda May-June 2016
STUDY ABROAD UGANDA-RWANDA MAY-JUNE 2016 GEG 103 – THE DEVELOPING WORLD AND HUM 107: CULTURES OF AFRICA On Campus Courses: March 14th to May 15th 2016 Travel to East Africa: May 21st to June 9th 2016 Harper College. International Studies and Programs Description 2 This is a unique opportunity to study abroad in Uganda and Rwanda, two countries on a continent that has led the world in economic growth over the last decade. This interdisciplinary study abroad experience will enrich your understanding of this rapidly changing part of the world. You will gain a firsthand introduction to the diverse environments, social issues, economic changes, and cultural traditions of Uganda and Rwanda. New perspectives on this region of the African continent will be gained in a variety of settings: including cities, agricultural areas, world renowned national parks, universities, and museums. Interaction with local experts and scholars will occur throughout the program. The field program will be preceded by an interdisciplinary study and introduction to the history, geography, and culture of Uganda and Rwanda in particular and East Africa in general. Program Overview 3 Instructors: Mukila Maitha and Dr. Richard Johnson Contact information: Mukila Maitha: J-268; [email protected] 847-925-6521 Dr. Richard Johnson: L-203; [email protected] 847-925-6429 Dates of Program: Class meetings at Harper College: will be conducted prior to the program between March 14th and May 15th to fulfill GEG 103 (Geography of the Developing World) or HUM 107 (Cultures of Africa) studies and requirements. st th Field Program in East Africa: May 21 through June 8 , 2016. -
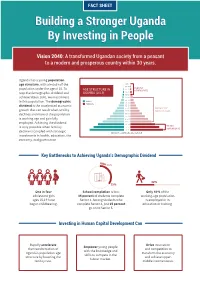
Building a Stronger Uganda Fact Sheet
FACT SHEET Building a Stronger Uganda By Investing in People Vision 2040: A transformed Ugandan society from a peasant to a modern and prosperous country within 30 years. Uganda has a young population age structure, with almost half the 85+ 80-84 ELDERLY population under the age of 15. To 75-79 AGE STRUCTURE IN DEPENDENTS UGANDA (2019) 70-74 reap the demographic dividend and 65-69 achieve Vision 2040, we must invest 60-64 55-59 MALES in this population. The demographic 50-54 FEMALES dividend is the accelerated economic 45-49 40-44 PRODUCTIVE growth that can result when fertility 35-39 WORKING AGES declines and more of the population 30-34 25-29 is working age and gainfully 20-24 15-19 employed. Achieving the dividend 10-14 YOUNG is only possible when fertility 5-9 0-4 DEPENDENTS decline is coupled with strategic PERCENT COMPRISING EACH GROUP investments in health, education, the economy, and governance. Key Bottlenecks to Achieving Uganda’s Demographic Dividend 25% 42% 35% One in four School completion is low: Only 42% of the adolescent girls 35 percent of students complete working-age population ages 15-19 have Senior 4. Among students who is employed or in begun childbearing. complete Senior 4, just 25 percent education or training. go on to Senior 5. Investing in Human Capital Development Can Rapidly accelerate Drive innovation Empower young people the transformation of and competition to with the knowledge and Uganda’s population age transform the economy skills to compete in the structure by lowering the and achieve upper- labour market. -

1 Communication 227/99, Democratic Republic of Congo V. Burundi, Rwanda and Uganda 1) Reference Details Jurisdiction: African C
Communication 227/99, Democratic Republic of Congo v. Burundi, Rwanda and Uganda 1) Reference Details Jurisdiction: African Commission of Human and Peoples’ Rights Date of Decision: 33 rd Ordinary Session, May 2003 Case Status: Concluded 2) Facts The case concerned a Communication made by the Democratic Republic of the Congo (DRC) to the African Commission alleging violations of the African Charter on Human and Peoples’ Rights (African Charter) and international law including the Geneva Conventions 1949 and Additional Protocols, the UN Charter and UN Declaration on Friendly Relations. It was also the first inter- state communication filed before the African Commission on Human and Peoples’ Rights (the Commission). The DRC alleged that armed forces from Burundi, Uganda and Rwanda had been occupying its border provinces in the eastern part of the country and committing mass violations of human rights and international law. Such alleged violations included the mass killing of civilians and the siege of a hydroelectric dam (civilian run) resulting in the cut off of electricity to homes, schools and hospitals – which led to the deaths of patients dependent on life support systems. The DRC further alleges, in particular against the Ugandan soldiers, the deliberate spread of HIV/AIDS amongst the local population by the perpetration of rape. Further allegations were the mass looting of civilian property and the natural mineral wealth in the region, as well as the forced movement of populations from the region into ‘concentration camps’ in Rwanda -

Ebola in the Context of Conflict Affected States and Health Systems
McPake et al. Conflict and Health (2015) 9:23 DOI 10.1186/s13031-015-0052-7 CASE STUDY Open Access Ebola in the context of conflict affected states and health systems: case studies of Northern Uganda and Sierra Leone Barbara McPake1,2*, Sophie Witter1, Sarah Ssali3, Haja Wurie4, Justine Namakula5 and Freddie Ssengooba5 Abstract Ebola seems to be a particular risk in conflict affected contexts. All three of the countries most affected by the 2014-15 outbreak have a complex conflict-affected recent history. Other major outbreaks in the recent past, in Northern Uganda and in the Democratic Republic of Congo are similarly afflicted although outbreaks have also occurred in stable settings. Although the 2014-15 outbreak in West Africa has received more attention than almost any other public health issue in recent months, very little of that attention has focused on the complex interaction between conflict and its aftermath and its implications for health systems, the emergence of the disease and the success or failure in controlling it. The health systems of conflict-affected states are characterized by a series of weaknesses, some common to other low and even middle income countries, others specifically conflict-related. Added to this is the burden placed on health systems by the aggravated health problems associated with conflict. Other features of post conflict health systems are a consequence of the global institutional response. Comparing the experience of Northern Uganda and Sierra Leone in the emergence and management of Ebola outbreaks in 2000-1 and in 2014-15 respectively highlights how the various elements of these conflict affected societies came together with international agencies responses to permit the outbreak of the disease and then to successfully contain it (in Northern Uganda) or to fail to do so before a catastrophic cost had been incurred (in Sierra Leone). -

From Uganda to the Congo and Beyond: Pursuing the Lord's Resistance Army
From Uganda to the Congo and Beyond: Pursuing the Lord's Resistance Army Ronald R. Atkinson DECEMBER 2009 INTERNATIONAL PEACE INSTITUTE Cover Photo: Men from the Ugandan ABOUT THE AUTHOR government local defense forces ride in the back of a militarized RONALD R. ATKINSON is Director of African Studies at the vehicle in Gulu, Uganda. July 31, University of South Carolina. He has lived and worked in 2003. © AP Photo/Marcus Kenya and Uganda in East Africa, Ghana in West Africa, Bleasdale. and South Africa. His major focus of research and writing is The views expressed in this paper the Acholi region and people in northern Uganda. represent those of the author and not necessarily those of IPI. IPI welcomes consideration of a wide ACKNOWLEDGEMENTS range of perspectives in the pursuit of a well-informed debate on critical IPI owes a great debt of thanks to the generous contribu - policies and issues in international tors to its Africa program. Their support reflects a affairs. widespread demand for innovative thinking on practical solutions to continental challenges. In particular, IPI and the IPI Publications Adam Lupel, Editor Africa program are grateful to the government of the Ellie B. Hearne, Publications Officer Netherlands. Suggested Citation The author is grateful to Adam Branch, Ed Carr, Denise Ronald R. Atkinson, “From Uganda Duovant, Sverker Finnström, and Eric Green for their astute to the Congo and Beyond: Pursuing and helpful comments on the penultimate draft. the Lord’s Resistance Army,” New York: International Peace Institute, December 2009. © by International Peace Institute, 2009 All Rights Reserved www.ipinst.org CONTENTS Executive Summary . -

Introduction Kenya, Tanzania, and Uganda Have Had A
EAC Introduction Member Countries Kenya, Tanzania, and Uganda have had a history of Burundi South Sudan cooperation that dates back to the early 20th century. The Kenya Tanzania Customs Union between Kenya and Uganda was formed in Rwanda Uganda 1917, and Tanzania joined in 1927. Burundi and Rwanda joined in 2009, and South Sudan joined in 2016. The EAC History has had 3 names since its conception; the East African High Commission from 1948 to 1961, East African Common 1917 - Customs union between Kenya and Uganda is Services Organization from 1961 to 1967, and then from formed. 1967 to 1977 the East African Community. In 1967 the East African Common Services Organization was superseded by 1927 - Tanzania joins the Customs Union. the East African Community, which aimed to strengthen the 1948 - EAC is named the East African High Commission. ties between the members through a common market, a 1961 - The name changes to East African Common common customs tariff, and a range of public serves to help Services Organization. achieve economic growth in the region. In 1977, the East African Community collapsed due to Kenya requesting more 1967 - The East African Common Services Organization is seats in the decision-making organs, disagreements superseded by the East African Community. between member countries, and the different economic 1977 - The East African Community collapses due systems between countries, primarily between Tanzania disagreements between member countries. and Kenya. The East African Community was re-established when its treaty was signed on November 30, 1999, and November 30, 1999 - The East African Community is came into force on July 7, 2000.