Centronuclear Myopathies Norma Romero, Marc Bitoun
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Centronuclear Myopathies Under Attack: a Plethora of Therapeutic Targets Hichem Tasfaout, Belinda Cowling, Jocelyn Laporte
CORE Metadata, citation and similar papers at core.ac.uk Provided by Archive Ouverte en Sciences de l'Information et de la Communication Centronuclear myopathies under attack: A plethora of therapeutic targets Hichem Tasfaout, Belinda Cowling, Jocelyn Laporte To cite this version: Hichem Tasfaout, Belinda Cowling, Jocelyn Laporte. Centronuclear myopathies under attack: A plethora of therapeutic targets. Journal of Neuromuscular Diseases, IOS Press, 2018, 5, pp.387 - 406. 10.3233/JND-180309. hal-02438924 HAL Id: hal-02438924 https://hal.archives-ouvertes.fr/hal-02438924 Submitted on 14 Jan 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Journal of Neuromuscular Diseases 5 (2018) 387–406 387 DOI 10.3233/JND-180309 IOS Press Review Centronuclear myopathies under attack: A plethora of therapeutic targets Hichem Tasfaouta,b,c,d, Belinda S. Cowlinga,b,c,d,1 and Jocelyn Laportea,b,c,d,1,∗ aDepartment of Translational Medicine and Neurogenetics, Institut de G´en´etique et de Biologie Mol´eculaire et Cellulaire (IGBMC), Illkirch, France bInstitut National de la Sant´eetdelaRechercheM´edicale (INSERM), U1258, Illkirch, France cCentre National de la Recherche Scientifique (CNRS), UMR7104, Illkirch, France dUniversit´e de Strasbourg, Illkirch, France Abstract. -

Myotubular Myopathy Caused by Multiple Abnormal Splicing Variants in the MTM1 RNA in a Patient with a Mild Phenotype
European Journal of Human Genetics (2012) 20, 701–704 & 2012 Macmillan Publishers Limited All rights reserved 1018-4813/12 www.nature.com/ejhg SHORT REPORT Myotubular myopathy caused by multiple abnormal splicing variants in the MTM1 RNA in a patient with a mild phenotype Nasim Vasli1,2,3,4, Vincent Laugel5, Johann Bo¨hm1,2,3,4,Be´atrice Lannes6, Vale´rie Biancalana1,2,3,4,7and Jocelyn Laporte*,1,2,3,4 Mutations impacting on the splicing of pre-mRNA are one important cause of genetically inherited diseases. However, detection of splice mutations, that are mainly due to intronic variations, and characterization of their effects are usually not performed as a first approach during genetic diagnosis. X-linked recessive myotubular myopathy is a severe congenital myopathy due to mutations in the MTM1 gene encoding myotubularin. Here, we screened a male patient showing an unusually mild phenotype without respiratory distress by western blot with specific myotubularin antibodies and detected a strong reduction of the protein level.The disease was subsequently linked to a hemizygous point mutation affecting the acceptor splice site of exon 8 of MTM1, proven by protein, transcript and genomic DNA analysis. Detailed analysis of the MTM1 mRNA by RT-PCR, sequencing and quantitative PCR revealed multiple abnormal transcripts with retention of a truncated exon 8, and neighboring exons 7 and 9 but exclusion of several other exons, suggesting a complex effect of this mutation on the splicing of non-adjacent exons. We conclude that the analysis of RNA by RT-PCR and sequencing is an important step to characterize the precise impact of detected splice variants. -

Affected Female Carriers of MTM1 Mutations Display a Wide Spectrum
Acta Neuropathol DOI 10.1007/s00401-017-1748-0 ORIGINAL PAPER Afected female carriers of MTM1 mutations display a wide spectrum of clinical and pathological involvement: delineating diagnostic clues Valérie Biancalana1,2,3,4,5 · Sophie Scheidecker1 · Marguerite Miguet1 · Annie Laquerrière6 · Norma B. Romero7,8 · Tanya Stojkovic8 · Osorio Abath Neto9 · Sandra Mercier10,11,12 · Nicol Voermans13 · Laura Tanner14 · Curtis Rogers15 · Elisabeth Ollagnon‑Roman16 · Helen Roper17 · Célia Boutte18 · Shay Ben‑Shachar19 · Xavière Lornage2,3,4,5 · Nasim Vasli2,3,4,5 · Elise Schaefer20 · Pascal Laforet21 · Jean Pouget22 · Alexandre Moerman23 · Laurent Pasquier24 · Pascale Marcorelle25,26 · Armelle Magot12 · Benno Küsters27 · Nathalie Streichenberger28 · Christine Tranchant29 · Nicolas Dondaine1 · Raphael Schneider2,3,4,5,30 · Claire Gasnier1 · Nadège Calmels1 · Valérie Kremer31 · Karine Nguyen32 · Julie Perrier12 · Erik Jan Kamsteeg33 · Pierre Carlier34 · Robert‑Yves Carlier35 · Julie Thompson30 · Anne Boland36 · Jean‑François Deleuze36 · Michel Fardeau7,8 · Edmar Zanoteli9 · Bruno Eymard21 · Jocelyn Laporte2,3,4,5 Received: 9 May 2017 / Revised: 24 June 2017 / Accepted: 2 July 2017 © Springer-Verlag GmbH Germany 2017 Abstract X-linked myotubular myopathy (XLMTM), a females and to delineate diagnostic clues, we character- severe congenital myopathy, is caused by mutations in the ized 17 new unrelated afected females and performed a MTM1 gene located on the X chromosome. A majority of detailed comparison with previously reported cases at the afected males die in the early postnatal period, whereas clinical, muscle imaging, histological, ultrastructural and female carriers are believed to be usually asymptomatic. molecular levels. Taken together, the analysis of this large Nevertheless, several afected females have been reported. cohort of 43 cases highlights a wide spectrum of clini- To assess the phenotypic and pathological spectra of carrier cal severity ranging from severe neonatal and generalized weakness, similar to XLMTM male, to milder adult forms. -

2021 Code Changes Reference Guide
Boston University Medical Group 2021 CPT Code Changes Reference Guide Page 1 of 51 Background Current Procedural Terminology (CPT) was created by the American Medical Association (AMA) in 1966. It is designed to be a means of effective and dependable communication among physicians, patients, and third-party payers. CPT provides a uniform coding scheme that accurately describes medical, surgical, and diagnostic services. CPT is used for public and private reimbursement systems; development of guidelines for medical care review; as a basis for local, regional, and national utilization comparisons; and medical education and research. CPT Category I codes describe procedures and services that are consistent with contemporary medical practice. Category I codes are five-digit numeric codes. CPT Category II codes facilitate data collection for certain services and test results that contribute to positive health outcomes and quality patient care. These codes are optional and used for performance management. They are alphanumeric five-digit codes with the alpha character F in the last position. CPT Category III codes represent emerging technologies. They are alphanumeric five-digit codes with the alpha character T in the last position. The CPT Editorial Panel, appointed by the AMA Board of Trustees, is responsible for maintaining and updating the CPT code set. Purpose The AMA makes annual updates to the CPT code set, effective January 1. These updates include deleted codes, revised codes, and new codes. It’s important for providers to understand the code changes and the impact those changes will have to systems, workflow, reimbursement, and RVUs. This document is meant to assist you with this by providing a summary of the changes; a detailed breakdown of this year’s CPT changes by specialty, and HCPCS Updates for your reference. -

Psykisk Utviklingshemming Og Forsinket Utvikling
Psykisk utviklingshemming og forsinket utvikling Genpanel, versjon v03 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC >x10 Andel av genet som har blitt lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering x10 er forventet dekning; faktisk dekning vil variere. Gen Gen (HGNC Transkript >10x Fenotype (symbol) ID) AAAS 13666 NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AARS 20 NM_001605.2 100% Charcot-Marie-Tooth disease, axonal, type 2N OMIM Epileptic encephalopathy, early infantile, 29 OMIM AASS 17366 NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCB11 42 NM_003742.2 100% Cholestasis, benign recurrent intrahepatic, 2 OMIM Cholestasis, progressive familial intrahepatic 2 OMIM ABCB7 48 NM_004299.5 100% Anemia, sideroblastic, with ataxia OMIM ABCC6 57 NM_001171.5 93% Arterial calcification, generalized, of infancy, 2 OMIM Pseudoxanthoma elasticum OMIM Pseudoxanthoma elasticum, forme fruste OMIM ABCC9 60 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 61 NM_000033.3 77% Adrenoleukodystrophy OMIM Adrenomyeloneuropathy, adult OMIM ABCD4 68 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 21396 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 21497 NM_014049.4 99% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM 89 NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS 90 NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL 92 NM_000018.3 100% VLCAD -

(12) Patent Application Publication (10) Pub. No.: US 2016/0281166 A1 BHATTACHARJEE Et Al
US 20160281 166A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0281166 A1 BHATTACHARJEE et al. (43) Pub. Date: Sep. 29, 2016 (54) METHODS AND SYSTEMIS FOR SCREENING Publication Classification DISEASES IN SUBJECTS (51) Int. Cl. (71) Applicant: PARABASE GENOMICS, INC., CI2O I/68 (2006.01) Boston, MA (US) C40B 30/02 (2006.01) (72) Inventors: Arindam BHATTACHARJEE, G06F 9/22 (2006.01) Andover, MA (US); Tanya (52) U.S. Cl. SOKOLSKY, Cambridge, MA (US); CPC ............. CI2O 1/6883 (2013.01); G06F 19/22 Edwin NAYLOR, Mt. Pleasant, SC (2013.01); C40B 30/02 (2013.01); C12O (US); Richard B. PARAD, Newton, 2600/156 (2013.01); C12O 2600/158 MA (US); Evan MAUCELI, (2013.01) Roslindale, MA (US) (21) Appl. No.: 15/078,579 (57) ABSTRACT (22) Filed: Mar. 23, 2016 Related U.S. Application Data The present disclosure provides systems, devices, and meth (60) Provisional application No. 62/136,836, filed on Mar. ods for a fast-turnaround, minimally invasive, and/or cost 23, 2015, provisional application No. 62/137,745, effective assay for Screening diseases, such as genetic dis filed on Mar. 24, 2015. orders and/or pathogens, in Subjects. Patent Application Publication Sep. 29, 2016 Sheet 1 of 23 US 2016/0281166 A1 SSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSS S{}}\\93? sau36 Patent Application Publication Sep. 29, 2016 Sheet 2 of 23 US 2016/0281166 A1 &**** ? ???zzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzz??º & %&&zzzzzzzzzzzzzzzzzzzzzzz &Sssssssssssssssssssssssssssssssssssssssssssssssssssssssss & s s sS ------------------------------ Patent Application Publication Sep. 29, 2016 Sheet 3 of 23 US 2016/0281166 A1 23 25 20 FG, 2. Patent Application Publication Sep. 29, 2016 Sheet 4 of 23 US 2016/0281166 A1 : S Patent Application Publication Sep. -

Myopathies Infosheet
The University of Chicago Genetic Services Laboratories 5841 S. Maryland Ave., Rm. G701, MC 0077, Chicago, Illinois 60637 Toll Free: (888) UC GENES (888) 824 3637 Local: (773) 834 0555 FAX: (773) 702 9130 [email protected] dnatesting.uchicago.edu CLIA #: 14D0917593 CAP #: 18827-49 Gene tic Testing for Congenital Myopathies/Muscular Dystrophies Congenital Myopathies Congenital myopathies are typically characterized by the presence of specific structural and histochemical features on muscle biopsy and clinical presentation can include congenital hypotonia, muscle weakness, delayed motor milestones, feeding difficulties, and facial muscle involvement (1). Serum creatine kinase may be normal or elevated. Heterogeneity in presenting symptoms can occur even amongst affected members of the same family. Congenital myopathies can be divided into three main clinicopathological defined categories: nemaline myopathy, core myopathy and centronuclear myopathy (2). Nemaline Myopathy Nemaline Myopathy is characterized by weakness, hypotonia and depressed or absent deep tendon reflexes. Weakness is typically proximal, diffuse or selective, with or without facial weakness and the diagnostic hallmark is the presence of distinct rod-like inclusions in the sarcoplasm of skeletal muscle fibers (3). Core Myopathy Core Myopathy is characterized by areas lacking histochemical oxidative and glycolytic enzymatic activity on histopathological exam (2). Symptoms include proximal muscle weakness with onset either congenitally or in early childhood. Bulbar and facial weakness may also be present. Patients with core myopathy are typically subclassified as either having central core disease or multiminicore disease. Centronuclear Myopathy Centronuclear Myopathy (CNM) is a rare muscle disease associated with non-progressive or slowly progressive muscle weakness that can develop from infancy to adulthood (4, 5). -

Prenatal Testing Requisition Form
BAYLOR MIRACA GENETICS LABORATORIES SHIP TO: Baylor Miraca Genetics Laboratories 2450 Holcombe, Grand Blvd. -Receiving Dock PHONE: 800-411-GENE | FAX: 713-798-2787 | www.bmgl.com Houston, TX 77021-2024 Phone: 713-798-6555 PRENATAL COMPREHENSIVE REQUISITION FORM PATIENT INFORMATION NAME (LAST,FIRST, MI): DATE OF BIRTH (MM/DD/YY): HOSPITAL#: ACCESSION#: REPORTING INFORMATION ADDITIONAL PROFESSIONAL REPORT RECIPIENTS PHYSICIAN: NAME: INSTITUTION: PHONE: FAX: PHONE: FAX: NAME: EMAIL (INTERNATIONAL CLIENT REQUIREMENT): PHONE: FAX: SAMPLE INFORMATION CLINICAL INDICATION FETAL SPECIMEN TYPE Pregnancy at risk for specific genetic disorder DATE OF COLLECTION: (Complete FAMILIAL MUTATION information below) Amniotic Fluid: cc AMA PERFORMING PHYSICIAN: CVS: mg TA TC Abnormal Maternal Screen: Fetal Blood: cc GESTATIONAL AGE (GA) Calculation for AF-AFP* NTD TRI 21 TRI 18 Other: SELECT ONLY ONE: Abnormal NIPT (attach report): POC/Fetal Tissue, Type: TRI 21 TRI 13 TRI 18 Other: Cultured Amniocytes U/S DATE (MM/DD/YY): Abnormal U/S (SPECIFY): Cultured CVS GA ON U/S DATE: WKS DAYS PARENTAL BLOODS - REQUIRED FOR CMA -OR- Maternal Blood Date of Collection: Multiple Pregnancy Losses LMP DATE (MM/DD/YY): Parental Concern Paternal Blood Date of Collection: Other Indication (DETAIL AND ATTACH REPORT): *Important: U/S dating will be used if no selection is made. Name: Note: Results will differ depending on method checked. Last Name First Name U/S dating increases overall screening performance. Date of Birth: KNOWN FAMILIAL MUTATION/DISORDER SPECIFIC PRENATAL TESTING Notice: Prior to ordering testing for any of the disorders listed, you must call the lab and discuss the clinical history and sample requirements with a genetic counselor. -

Recurrent Pregnancy Loss Precision Panel Overview Indications Clinical
Recurrent Pregnancy Loss Precision Panel Overview Recurrent Pregnancy Loss (RPL) is one of the most common obstetric complications, affecting more than 30% of conceptions. These can occur during preimplantation, pre-embryonic, embryonic, early fetal, late fetal and stillbirth. An important number of losses are due to genetic abnormalities, nonetheless 50% of early pregnancy losses have been associated with chromosomal abnormalities. The majority are due to de novo non-disjunctional events during meiosis and balanced paternal translocations. Traditionally, the assessment of recurrent pregnancy loss was based on karyotyping techniques. However, advances in molecular genetic technology have provided an array of information regarding genetic causes and risk factors for pregnancy loss. One of the most innovative techniques with a significant role in RPL is preimplantation genetic testing in in vitro fertilization cycles. The Igenomix Recurrent Pregnancy Loss Precision Panel can be used to make a directed and accurate differential diagnosis of inability to carry out a full pregnancy ultimately leading to a better management and achieve a healthy baby at home. It provides a comprehensive analysis of the genes involved in this disease using next-generation sequencing (NGS) to fully understand the spectrum of relevant genes involved. Indications The Igenomix Recurrent Pregnancy Loss Precision Panel is indicated for those patients the following manifestations: - Inability to conceive after 1 year of unprotected intercourse - Family history of infertility - Personal history of recurrent miscarriages - Family history of recurrent miscarriages - Previous failed IVF cycles - Other failed assisted reproductive technology (ART) treatments Clinical Utility The clinical utility of this panel is: - The genetic and molecular confirmation for an accurate clinical diagnosis of a symptomatic patient. -

Blueprint Genetics Comprehensive Muscular Dystrophy / Myopathy Panel
Comprehensive Muscular Dystrophy / Myopathy Panel Test code: NE0701 Is a 125 gene panel that includes assessment of non-coding variants. In addition, it also includes the maternally inherited mitochondrial genome. Is ideal for patients with distal myopathy or a clinical suspicion of muscular dystrophy. Includes the smaller Nemaline Myopathy Panel, LGMD and Congenital Muscular Dystrophy Panel, Emery-Dreifuss Muscular Dystrophy Panel and Collagen Type VI-Related Disorders Panel. About Comprehensive Muscular Dystrophy / Myopathy Muscular dystrophies and myopathies are a complex group of neuromuscular or musculoskeletal disorders that typically result in progressive muscle weakness. The age of onset, affected muscle groups and additional symptoms depend on the type of the disease. Limb girdle muscular dystrophy (LGMD) is a group of disorders with atrophy and weakness of proximal limb girdle muscles, typically sparing the heart and bulbar muscles. However, cardiac and respiratory impairment may be observed in certain forms of LGMD. In congenital muscular dystrophy (CMD), the onset of muscle weakness typically presents in the newborn period or early infancy. Clinical severity, age of onset, and disease progression are highly variable among the different forms of LGMD/CMD. Phenotypes overlap both within CMD subtypes and among the congenital muscular dystrophies, congenital myopathies, and LGMDs. Emery-Dreifuss muscular dystrophy (EDMD) is a condition that affects mainly skeletal muscle and heart. Usually it presents in early childhood with contractures, which restrict the movement of certain joints – most often elbows, ankles, and neck. Most patients also experience slowly progressive muscle weakness and wasting, beginning with the upper arm and lower leg muscles and progressing to shoulders and hips. -

Clinical, Pathological and Genetic Characteristics of Autosomal Dominant Inherited Dynamin 2 Centronuclear Myopathy
MOLECULAR MEDICINE REPORTS 13: 4273-4278, 2016 Clinical, pathological and genetic characteristics of autosomal dominant inherited dynamin 2 centronuclear myopathy XINHONG LIU1,2, HUAMIN WU3, JIAN GONG4, TAO WANG2 and CHUANZHU YAN1 1Department of Neurology, Qilu Hospital of Shandong University, Jinan, Shandong 250012; Departments of 2Neurology, 3Radiology and 4Digestive System, Tai'an Central Hospital, Tai'an, Shandong 271000, P.R. China Received February 21, 2015; Accepted December 18, 2015 DOI: 10.3892/mmr.2016.5047 Abstract. The aim of the present study was to report on a the myotubularin 1 gene (MTM1) (2). A third gene, associ- family with pathologically and genetically diagnosed auto- ated with autosomal recessive inherited CNM, is bridging somal dominant inherited centronuclear myopathy (CNM). In integrator 1 (3). addition, this study aimed to investigate the clinical, patho- Previous studies on DNM2-associated CNM logical and molecular genetic characteristics of the disease. (DNM2 -CNM) cases have demonstrated that it can range from This pedigree was traced back three generations, four patients severe neonatal onset to mild adult onset, and the severity of underwent neurological examination, two patients underwent the clinical manifestations vary (4-7). The majority of patients muscle biopsy, and eight family members were subjected to exhibit distal or proximal muscle weakness as a first symptom, dynamin 2 (DNM2) gene mutation analysis. DNM2 mutations presenting as general muscle weakness, eyelid drooping, were detected in seven family members, of which four patients extraocular muscle paralysis, high arch palate, contracture of exhibited DNM2 mutation‑specific clinical and pathological the Achilles tendon, trismus, and reduction or lack of tendon features. -
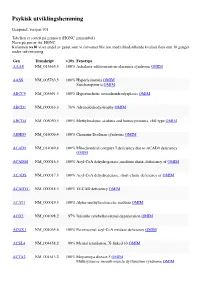
Psykisk Utviklingshemming
Psykisk utviklingshemming Genpanel, versjon v01 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC Kolonnen >x10 viser andel av genet som vi forventer blir lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering Gen Transkript >10x Fenotype AAAS NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AASS NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCC9 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 NM_000033.3 76% Adrenoleukodystrophy OMIM ABCD4 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 NM_014049.4 100% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL NM_000018.3 100% VLCAD deficiency OMIM ACAT1 NM_000019.3 100% Alpha-methylacetoacetic aciduria OMIM ACO2 NM_001098.2 97% Infantile cerebellar-retinal degeneration OMIM ACOX1 NM_004035.6 100% Peroxisomal acyl-CoA oxidase deficiency OMIM ACSL4 NM_004458.2 99% Mental retardation, X-linked 63 OMIM ACTA2 NM_001613.2 100% Moyamoya disease 5 OMIM Multisystemic smooth muscle dysfunction syndrome OMIM Gen Transkript >10x Fenotype ACTB NM_001101.3 100% ?Dystonia, juvenile-onset OMIM Baraitser-Winter syndrome 1 OMIM ACTG1 NM_001614.3 100% Baraitser-Winter syndrome 2 OMIM Deafness, autosomal dominant 20/26 OMIM ACVR1 NM_001105.4 100% Fibrodysplasia ossificans